A lot has changed over the past few decades in the way clinical trials are conducted. Advances in data management systems, risk-based monitoring, pharmacovigilance, electronic databases, and application of complex statistical techniques for data analysis are some of the ways in which progress in clinical trials have been made. Until the mid-1990s most big- and mid-sized pharma companies carried out their own clinical trials to place their new chemical entity (NCE) on the market.
However, a gamechanger that the clinical trial industry has seen particularly in the past few decades is the advent and rise in ‘outsourcing’ of trial activities. The term ‘outsourcing’ from a clinical trial perspective refers to the delegation of duties involved in the conduct of trials and related activities by the pharmaceutical or medical device company (sponsor) to contract research organizations (CROs).
Contract research organizations (CROs) are service organizations that provide support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. Today most activities that can be potentially done by a pharmaceutical company can be outsourced to CROs. These activities include preclinical testing of drugs or devices, synthesis of compounds and biochemical assays, and clinical trial services.
Clinical CROs focus on clinical trial services such as medical writing, data analysis, obtaining regulatory approvals and regulatory filing (1). The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) guideline defines CRO as a person or organization (commercial, academic, or other) contracted by the sponsor to perform one or more of a sponsor’s trial-related duties and functions. (2). Figure 1 shows the spectrum of activities that can be conducted by a clinical CRO.
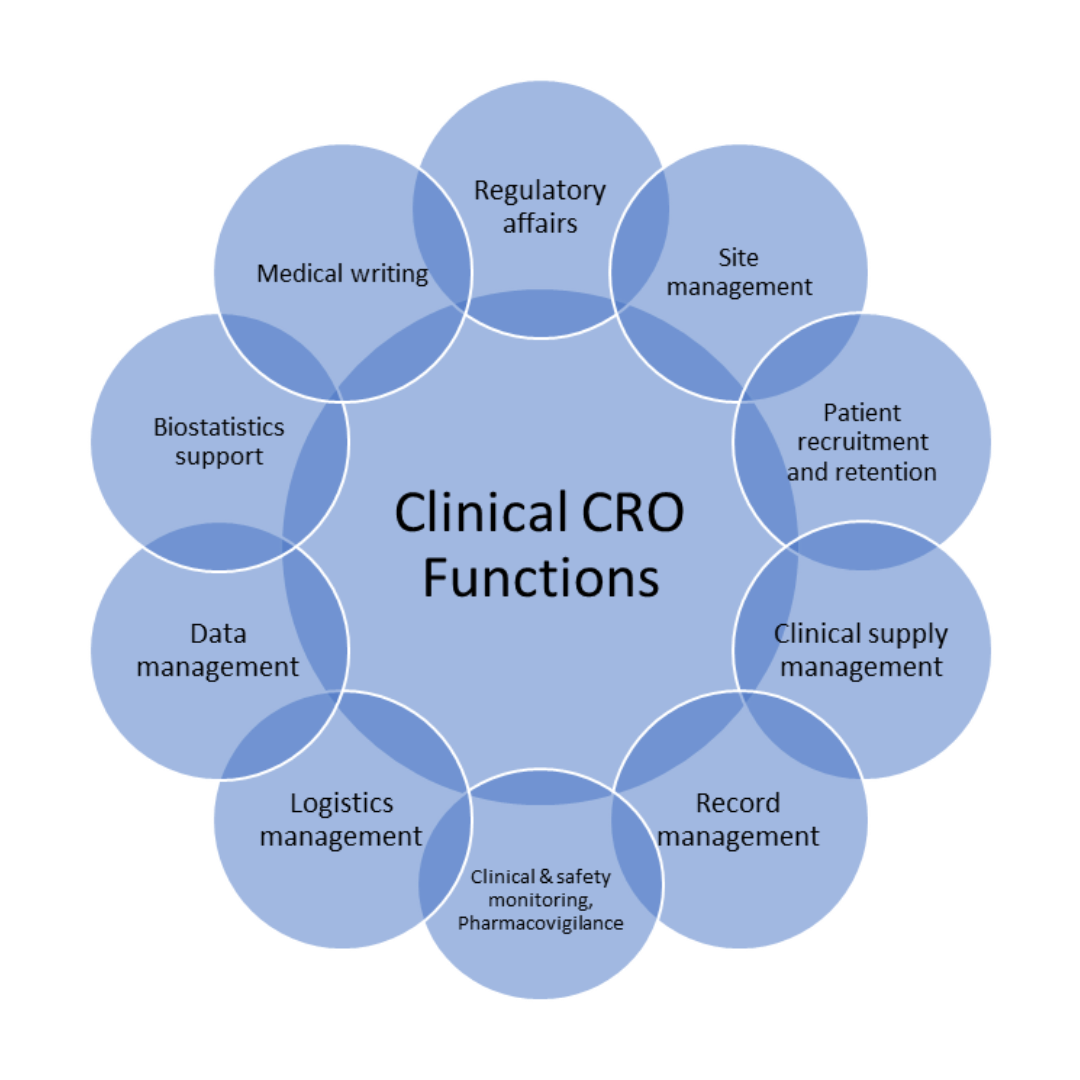
Figure 1. Outsourced activities to a clinical CRO
An important indicator of the adoption of the outsourcing model by pharmaceutical companies is the projected rise in the CRO services market from USD 53.2 billion in 2021 to USD 86.5 billion by 2026 which is primarily due to an increase in research and development (R&D) activities and clinical trials, and increased prevalence of rare and orphan diseases (3). The coronavirus outbreak in 2020 also led to increased pharmaceutical company-CRO partnerships to expedite R&D activities for development of vaccines, drugs, and diagnostics (3). An analysis in 2017 showed that on an average 64% of clinical development services (including early phase and Phase I-IV clinical trials, clinical data management) are outsourced with only 38-40% carried out in in-house proving that the outsourcing and strategic alliances are being widely used in the clinical trial arena (4). The US government estimated that approximately one-third of trials sponsored by the 20 largest pharmaceutical companies were conducted in foreign sites (5).
Drivers for clinical trial outsourcing from a US perspective: In-house vs Outsource
The key motivators for clinical trial outsourcing for large and mid-size pharmaceutical companies include downsizing and cost-cutting, CRO expertize in a particular therapeutic area or market in which the company has lack of experience regarding regulatory processes, operational expertize , and ability to use manpower for other activities during periods of increased workload.
- Outsourcing to CROs helps retain employees that are needed for other activities while decreasing the workforce and thus saving on salaries, overhead expenses, and infrastructure costs. This helps pharmaceutical companies save a considerable amount of money and decrease the burden on employees particularly at times where there is an increased workload.
- With the globalization of clinical trials and the need to perform multinational center trials often in countries where the company has little or no expertize regarding the regulatory landscape, CROs can help by providing local vendors to coordinate
trial-related activities. It is usually not feasible for sponsors to take on responsibilities for all activities and CROs can help bridge the gap between sponsors and local regulatory authorities to facilitate smooth functioning of the clinical trial and help hasten drug approval. - CROs can provide knowledge and expertize in therapeutic areas that the sponsor may not necessarily have which can allow for better management and design of the clinical trial. Additionally, CROs can also help recruit key opinion leaders (KOLs), physicians, and provide access to trial sites that are trained and experienced in a particular therapeutic area.
- CROs can provide access to ethnically diverse patient pools in different geographical locations which is necessary in some trials to understand drug efficacy.
From the perspective of US-based companies, outsourcing of clinical trials to other locations is largely driven by factors such challenges in patient recruitment and retention, high costs and lengthy regulatory timelines.
- Patient recruitment to obtain a suitable population size is necessary to obtain results from a trial that are both clinically and statistically meaningful. The inability to recruit patients that represent diverse classes such as women, ethnic minorities, and elderly and pediatric populations can result in delays in drug approvals. In the US, low populations of test subjects and unwillingness to volunteer is based on hesitation on the part of patients and their physicians to participate in trials due to increased workload and costs. The limited availability of KOLs willing to volunteers in trials is also lower in the US. Furthermore, it may be increasingly difficult to find appropriate subjects especially in cases of rare diseases or conditions in which the screening failure rate is high due to smaller patient pool.
- An obvious reason that companies may choose to conduct trials outside the US is cost. Recruitment, staff, administrative, insurance, resource, and infrastructure costs are much higher in the US compared to developing countries making outsourcing an attractive financial option. It is estimated that conducting a clinical trial in India costs approximately 60% less than in the US which is a significant financial advantage.
- Outsourcing helps pharmaceutical companies navigate complex regulatory pathways that can increase timelines for drug approval while placing a significant financial burden on the company. Multicentre trials benefit from local CROs that understand the regulatory requirements of the region. Since the process for drug approval by the US Food and Drug Administration (FDA) is a lengthy process, outsourcing can help shorten timelines as the CROs can facilitate faster approvals.
Although conducting clinical trials outside the US represents an attractive option both financially and logistically for pharmaceutical companies some challenges remain with the outsourcing model. Regulatory requirements such as Institutional Review Board (IRB) approvals, import licensing, and contract negotiations can differ between countries even in the same region making it necessary to choose an appropriate CRO that has a thorough understanding of these processes. It is important to ensure that the standard of care that the subjects receive in developing countries is on par with what would be provided to subjects in the US.
Protection of data integrity is also a concern when trials are carried out in rural areas along with poor infrastructure and logistics which may hamper transportation of the investigational drug and patient samples compromising trial data. Inability to adequately monitor clinical trials by local Institutional Review Boards (IRBs) due to financial constraints in developing countries may jeopardize participant safety. Furthermore, all studies carried out at foreign sites must comply with FDA regulations, be carried out in accordance with Good Clinical Practice (GCP) guidelines, and FDA should be able to validate the data through an onsite inspection. The benefits and risks associated with outsourcing should be evaluated when considering outsourcing.

Tips to select an appropriate CRO partner
The successful conduct and completion of a clinical trial in a timely and cost-effective manner hinges on the selection of a CRO by the sponsor that meets their needs. Some points to keep in mind when vetting CROs include:
- CRO experience in the therapeutic or geographic area where the clinical trial is to be conducted, ability to handle roadblocks that may occur such as site selection, patient recruitment and retention, and data management, relationships with KOLs and sites for conducting the trial, more information about the CRO can be obtained from client references.
- Quality management systems of the CRO-appropriate training of staff on quality management to comply with regional and global regulations, transparency of operating systems related to activities such as data security, and certificates on quality assurance should be provided by the CRO.
- CRO size: consideration of the CRO size is important depending on whether the sponsor is a small, mid, or big pharmaceutical company. Large CROs may be experienced but may be more costly and will not be able to give much attention to the project whereas smaller CROs can provide customized services with a greater focus on the client.
- Price of services: this is one of the most important factors as all pharmaceutical companies have a budget allocated to clinical trials. In addition, a breakdown of costs is necessary for each activity as bundled costs may not consider any unforeseen expense which could accumulate.
- Employee experience, training, qualification and CRO staff turnover are pivotal to the success of a trial.
- Accessibility: an appointed point of contact or a team that the sponsor can communicate with easily in case of any problems is essential to avoid major hurdles that can jeopardize trials.
Management of outsourced trials by sponsor
To avoid misunderstandings and maintain a healthy sponsor-CRO relationship, it is important that CROs and their outsourced activities are appropriately managed. Although a clinical trial may be outsourced to a CRO, FDA regulations require that the sponsor is responsible for the oversight of the trial. Some ways in which sponsors can manage CROs are:
- Develop comprehensive contractual agreements between the sponsor and CRO that outlines activities and responsibilities to be performed by each entity in a detailed manner. This contract should include financial, logistical, and legal responsibilities as well as audits and meeting schedules, quality measures, and schedules for completion of tasks and deliverables. This is a time-consuming task but should be outlined at the outset to avoid confusion and unnecessary delays.
- Creating a statement of work that defines the roles and responsibilities of the sponsor and CRO at each stage of the clinical trial will help avoid replication of activities by either party and allow the sponsor to take full benefit of the capabilities of the CRO. Every task along with a succinct description should be presented by the sponsor based on their expectations from the CRO without leaving anything open for interpretation.
- Schedules are necessary for timely completion of goals and milestones and can be provided in the form of Gantt chart for the project. Agreement on timelines is important.
- Regular meetings either in-person or virtually should be conducted and any problems or unforeseen issues should be discussed. It is important to keep the lines of communication open between the sponsor and CRO by appointing a person/team to be available for discussions if the need arises.
- A thorough understanding of the sponsors or CROs standard operating procedures (SOPs) is important. If the sponsors SOPs are being used it is necessary to maintain a training record to ensure that the CROs employees are well-versed with the SOPs. Staff should also be kept updated with any new quality system or GCP guidelines and these records should also be documented.
- Make sure ‘must-have’ expectations are specified to the CRO as time and resources are usually limited which will help CROs to avoid focusing on smaller, insignificant tasks.
- Sponsors should consider performing audits at trial sites to ensure compliance of the CRO with quality standards and documentation and gain an understanding at the ground level. It helps the sponsor understand whether the CRO staff can tackle problems and if the necessary corrective and preventative actions (CAPA) were taken. The timing of an audit is important as it should be done when enough subjects are enrolled but enough time is still left so any necessary changes can be made.
- Maintaining a professional relationship by understanding staff in each team and their expertize is necessary to ensure the success of the sponsor and CRO. Communication, trust, and agreement are key factors that should be kept in mind during any project.
US FDA oversight of outsourced clinical trials
In addition to management of CROs and outsourced activities by sponsors, the US FDA is responsible for overseeing the operations of clinical trials, both domestic and foreign, that are conducted in support of a future application for a new drug approval in the US. The FDA’s Office of Regulatory Affairs (ORA) has a Bioresearch Monitoring (BIMO) program which is implemented through multi-center compliance programs to ensure protection of rights, safety, and welfare of human subjects involved in FDA-regulated clinical studies (6).
ORA inspectors are required to conduct inspections to ensure:
- Compliance with applicable statutory and regulatory requirements
- Assure reliability of the study data
- Ensure that ethical and scientific standards are maintained during the clinical study
All activities even if outsourced to a CRO must be carried out in accordance with the protocol and investigational plan, GCP, and applicable FDA regulations. Inspection of CRO for outsourced activities by ORA investigators include (6):
- Evaluations of SOPs and work instructions developed by the sponsor and criteria for selection of CRO based on compliance with FDA regulations and GCP standards and provision of preferred vendor lists (if any).
- Detailed analysis of written agreements (master service agreements, statements of work and quality agreements) for critical steps in the trial to determine if roles, tasks, and responsibilities of sponsor and CRO are clearly defined, and which party is responsible for final decision-making for various steps of the trial.
- Determination of CRO employee qualifications and training records.
- Review of SOPs of either the sponsor or CRO depending on which one was followed including any deviation plans and records in case deviations occurred for documenting and rectifying them (CAPA). SOPs to be studied include those for audits, communications plans, escalation plans, and contingency plans.
- Review of sponsor-CRO communications to determine frequency, type, focus, and documentation of meetings.
- Ensure provision of protocol-specific training to CRO.
- Determination of sponsor’s oversight of activities outsourced to CRO through audit reports, if necessary.
The basic tenet of clinical trials is to protect the safety and welfare of human subjects along with providing meaningful data to support the efficacy of new drugs. This makes it important for CROs to comply with FDA regulations and GCP guidelines to facilitate approval of new drugs in the US market. Outsourcing clinical trials can be a win-win situation for the sponsor and CRO if there is adequate oversight and management of CROs by sponsors and FDA and mutual trust.
Need Support to Conduct your Clinical Trials in USA?
We’d be delighted to assist you in conducting your clinical trials in USA
Connect with our experienced staff in USA today!!
Provide details of your requirements by clicking on the given link below and our professional team of clinical research experts will get in touch with you right away.
References
- Landhius E. The rise of outsourcing. Nature 2018; 556: 263.
- E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry, March 2018.
- Contract Research Organization Services Market – Global Forecast to 2026 | Markets and Markets
- PAREXEL Biopharmaceutical Sourcebook 2016/2017, Beroe Analysis
- The Ethical Implications of the Global Outsourcing of Clinical Research – Public Citizen
- Food and Drug Administration Compliance Program Chapter 48: Bioresearch Monitoring (Program # 7348.810)






