Drug development strategy for US FDA
It takes a staggering 12 years on an average for a drug to travel from the laboratory bench to your bedside! This is coupled with up to approximately $1 billion in research and development costs that a pharmaceutical company needs to spend to bring its potential molecule to market. Furthermore, only about 10% of drugs that enter Phase I clinical trials are granted marketing approval by the Food and Drug Administration (US FDA). FDA is the regulatory body that handles drug approvals in the United States and has the world’s most stringent standards for approving drugs. The drug development process is well-defined and compartmentalized into logical stages starting from discovery to commercial use enabling companies to utilize this as roadmap when developing a drug.
The drug development process in the US is commonly divided below 5 stages:
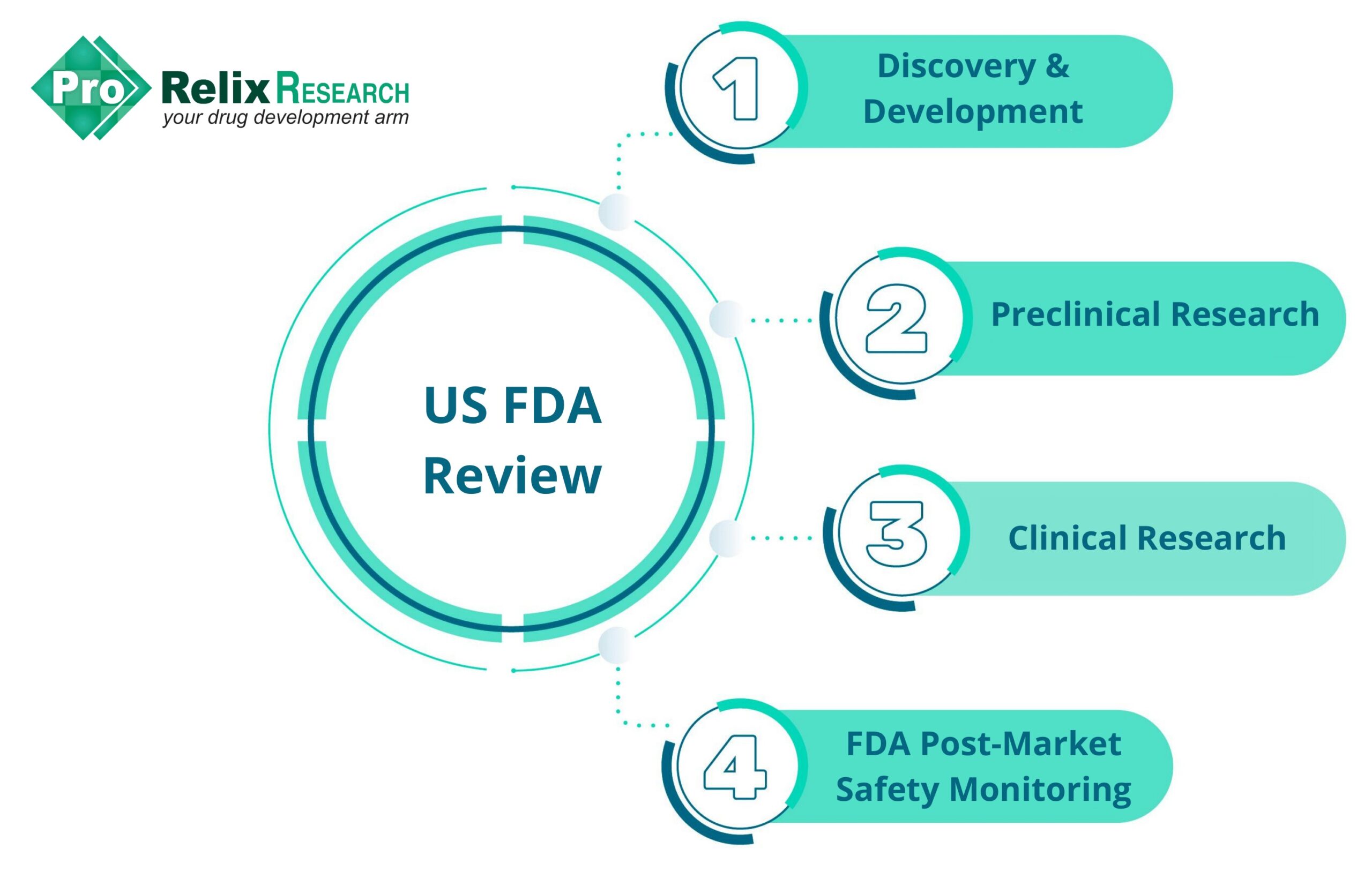
The various activities in each stage of the drug development process are as follows:
- Discovery and Development: Target and hit identification and optimization, assay development and validation, lead optimization, ADME, mechanism of action, dose and route of administration, drug interactions, effects in subgroups (gender, age, ethnicity, etc.)
- Preclinical research: In vivo animal studies, in vitro and ex vivo assays, formulation optimization, bioavailability, drug delivery, dose findings range
- Clinical research: Phase I (20-100 healthy volunteers or people with disease/condition, safety and dosage), Phase II ( ̴100 people with disease/condition, efficacy and side effects), Phase III (300-3000 volunteers with disease/condition, efficacy and monitoring of adverse reactions), Phase IV (several thousand volunteers who have disease/condition, safety, and efficacy)
- FDA review: New Drug Application (NDA), Abbreviated New Drug Application (ANDA), Biologics License Application (BLA) is filed by the company, FDA review, FDA approval
- FDA post-market safety monitoring: Collection of data on adverse events and untoward effects of drug
Of all stages in drug development, the clinical research stage is often the most laborious, expensive, and time-consuming and can last up to 7 years. Additionally, only 25-30% of drugs make their way from Phase I to Phase III trials.
What is the reason for this high attrition rate?
Often, regulatory agencies are blamed for failure of drugs to reach the market. However, poor planning (or in some cases, no clinical development plan at all) by pharmaceutical companies also contributes to failure to achieve marketing approval.
So, what can pharmaceutical companies do to boost their chances of success?
The answer is simple: develop a Target Product Profile (TPP), the heart of the drug development strategy
The adage “Well begun is half done” is usually a good point for pharmaceutical companies to consider when embarking on their drug development journey, which typically begins with the development of a TPP.
In 2007, the FDA released a draft guidance recommending the use of TPP as a strategic development tool to facilitate and streamline discussions between the Sponsor and the FDA, minimize the risk of late-stage drug failure, and allow for optimal safety and efficacy data to be available in a timely manner. Breder et al. showed that drugs and biologics approved by the FDA that used TPPs were associated with more efficient regulatory review times.
The TPP is basically a dynamic blueprint for the drug development process and provides a statement of the overall intent of the drug development program. Commercial goals of the product, pharmacology, and practicalities of clinical development should be considered when developing a TPP. Input from various stakeholders including regulatory, clinical, preclinical, marketing, research and development (R&D), and commercial teams is essential for formatting a TPP.
A TPP for a drug product generally includes the following:
- Indications and usage
- Dosage and administration
- Dosage forms and strengths
- Contraindications
- Warnings and precautions
- Adverse reactions
- Drug interactions
- Use in specific populations
- Drug abuse and dependence
- Overdosage
- Description
- Clinical pharmacology
- Nonclinical toxicology
- Clinical studies
- References
- Storage and handling conditions
- Patient counseling information
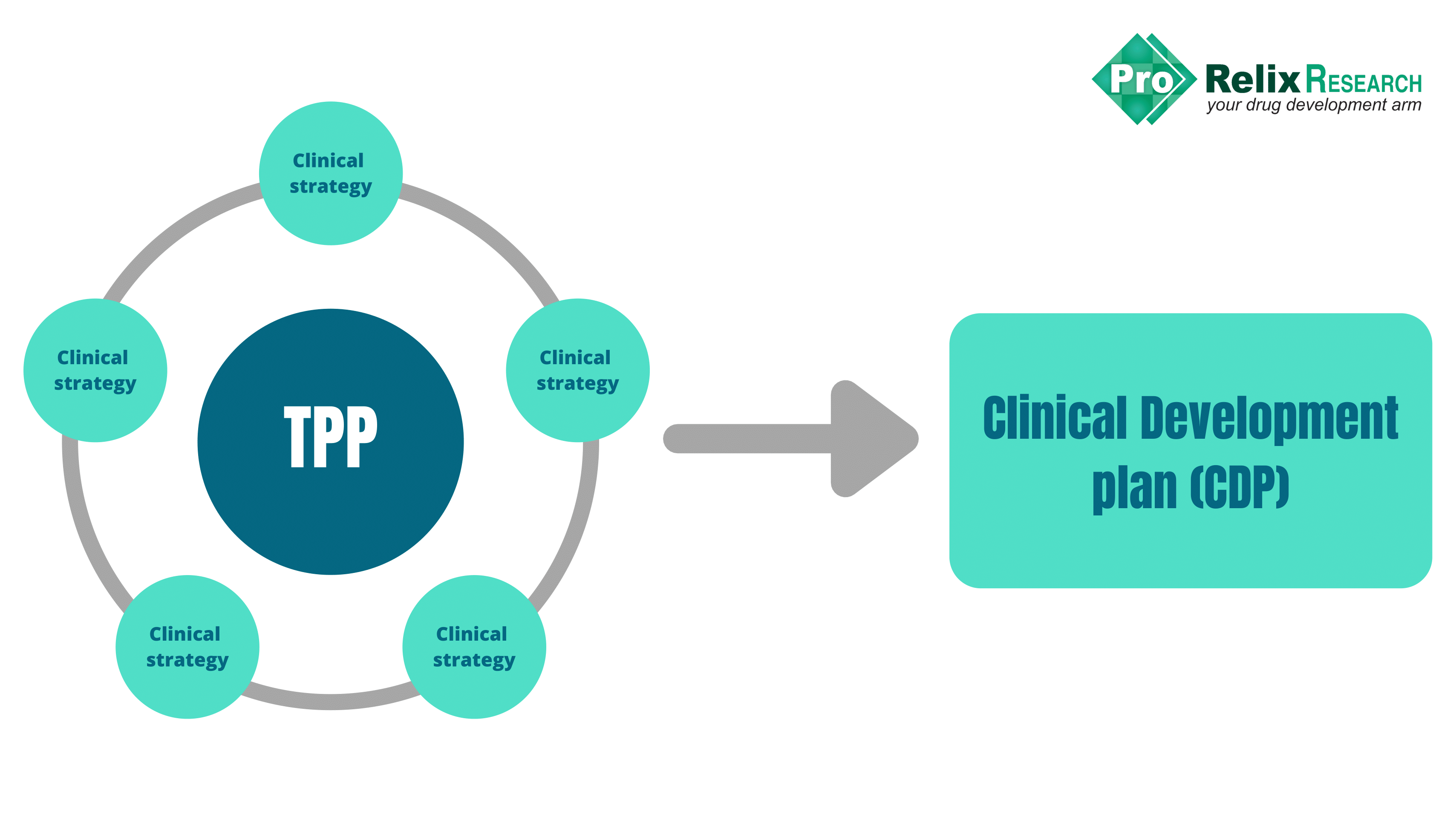
Clinical Development Plan (CDP)
Various strategies and plans are centered around the TPP that culminate in the clinical development plan (CDP). The CDP is founded on the goals stated in the TPP and presents the plan to achieve them.
The clinical development plan (CDP) should include:
Regulatory strategy
In addition to a thorough understanding of the regulatory landscape of the country in which the drug product is intended to be marketed, a comprehensive regulatory plan is essential for the smooth movement of the product through approval phases. A regulatory strategy should include:
- Pathway for regulatory approval
- Differentiating features of the product compared to other products in terms of labeling
- Guidelines, both global and local, that are applicable to the drug product development program
- Target timelines for regulatory submissions at different stages of product development (Pre-IND and NDA submissions)
- Well-developed plan for interaction with regulatory agencies/health authorities-what needs to be submitted at each stage, potential questions
- A thorough understanding of the format for regulatory submissions (electronic format as per FDA guidance document released in July 2021.
- Category of regulatory submission depending upon product characetristics: Investigational New Drug (IND) application or New Drug Application (NDA) regulatory pathway (505 (b)(1) or 505(b)(2))?
- Due diligence on regulatory pathway for similar drugs and their stage of development and approval
- Options available for registration such as fast-track approvals, orphan diseases, etc.
- Information on studies and data needed at each stage of drug product development for regulators to allow progress to the next stage
Nonclinical studies
Data from nonclinical studies are used to inform the design of clinical trials and is needed for regulators to ensure that drug is suitable to test in humans. These studies involve in vitro or ex vivo studies and animal studies and are carried out in accordance with ICH and Good Laboratory Practice (GLP) guidelines. Nonclinical studies plan should include:
- Choice of animal species (rodent and non-rodent)
- Tissue or cell culture methods for ex vivo studies
- Dose selection, frequency, and route of administration
- Analytical methods and validation parameters
- Toxicity studies-reproductive, carcinogenicity studies, genotoxicity, etc. and single-dose or multiple-dose studies
- Design of pharmacokinetic (PK) studies and ADME studies
Clinical studies
The clinical research phase (Phase I, II, and III studies) is the most laborious, expensive, and time-consuming step in the clinical development program. Despite the large investment in time and money spent on this phase, only 25-30% of drugs move from Phase I to Phase III. Clinical studies are essential to ensure the safety and efficacy of the drug in humans which is pivotal to its chances of success and therefore sufficient details for each phase are necessary.

The clinical trials design for each phase should include the following details:
- Study participants (gender, age, ethnicity, co-morbidities, etc.)
- Subject inclusion and exclusion criteria
- Trial design (parallel, crossover, factorial)
- Subject size calculation
- Control group (placebo, no comparator, active control)
- Design based on hypotheses (superiority, noninferiority, equivalence)
- Study endpoints: safety, efficacy, PK/PD parameters, patient-reported outcomes, biomarkers
- Dose and frequency of administration
- Dosage form and strength, route of administration
- Duration of treatment
- Resources needed for trial management and clinical supplies
- Schedule or timeline for completion of studies
- Resource allocation and investments
- Statistical analysis plan (details on type of statistical tests to be used, significance levels, software details)
- Risk evaluation and management strategies (REMS) and pharmacovigilance plans to monitor any post-approval safety concerns
Chemistry, manufacturing, and controls (CMC) studies
CMC activities ensure that a drug or product is safe, effective, and consistent between batches. These activities encompass characterization of the drug, manufacturing processes, drug product development, and scale-up to ensure that a reproducible molecule is available for clinical studies and commercial supply.
The following items should be included in a CMC plan:
- Drug and product development: synthetic routes to produce the drug, description of the chemical and physical properties of the drug such as pKa, solubility, melting points, spectral properties, log P (lipophilicity), polymorphs, etc., analytical method development and validation for quantification of drug and impurities or related substances, raw materials, drug, and excipient(s) sourcing and vendor supplies, formulation development: dose strength, formulation, and route of administration, stability protocols
- Quality related activities: quality control and in-process and finished drug/product inspections and tests, validation activities (sterility, cleaning), audit plans, quality management system development
- Clinical trial material: packaging and labeling of clinical trial material, storage, and transport conditions for clinical trial material
- Manufacturing process: validation, supply of labeling, vendor qualifications, scale-up and technology transfer
Commercial strategy
Although obtaining regulatory approval for a drug is a major feat for a pharmaceutical company it does not necessarily ensure commercial success in terms of sales and return on investment. Barriers to commercial success include competitive landscape and pricing, access challenges, and increased expectations by patients, physicians, and caregivers. A commercial strategy helps facilitate interactions with regulatory authorities and serves as a business tool for discussions with investors, partners, employees, physicians, and payors.
A comprehensive commercial strategy should include:
- A thorough analysis of the competitive landscape of the product with existing treatments in terms of efficacy, adverse effects, costs, patients, and physicians’ satisfaction
- Product differentiation in terms of outcomes and quality
- Information on pricing and reimbursement
- Understanding clinical and market needs (market size)
- Market research on optimal characteristics of the drug from key opinion leaders such as physicians and payors
- Health economic aspects such a trial endpoint, and real-world evidence (patient registries and databases) to ascertain the drugs economic benefit
The approval and commercial success of a new drug is an interplay of several factors: clinical, nonclinical, commercial, regulatory, and CMC. Starting off a drug development program ‘right’ with the construction of a TPP sets a strong foundation for further strategic development and planning in the above areas to allow for a smooth launch of a drug. This not only assures interaction with regulatory authorities but also keeps internal company functions aligned with meeting predetermined product goals.
Need Support to Conduct your Clinical Trials in USA?
We’d be delighted to assist you in conducting your clinical trials in USA
Connect with our experienced staff in USA today!!
Provide details of your requirements by clicking on the given link below and our professional team of clinical research experts will get in touch with you right away.
References:
- Woulters, O.J., McKee, M., Luyten, J. Estimated research and development investment to bring a new medicine to market, 2009-2018 JAMA 2020. 323: 844-853.
- https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
- Breder, C.D., Du, W., Tyndall, A. What’s the regulatory value of a target product profile? Trends in Biotechnology 2017. 35 (7): 576-579.
- Singh, G. Target Product Profile and Clinical Development Plan. Pharmaceutical Medicine and Translational Clinical Research 2018. 65-80
- Clinical Development Plan Guide 110613 (biostrategics.com)
- Target Product Profile-A Strategic Development Process Tool. Draft Guidance March 2007





