According to the World Health Organization (WHO), cancer is the leading cause of death worldwide, with a death rate of one in six in 2020 (1). Aside from the high mortality rate and morbidity associated with cancer, it also negatively impacts the quality of life and poses a significant financial burden on patients and payers making it imperative to develop effective treatments for the disease. According to Global Cancer Observatory (GLOBACAN), the United States accounted for 13.3% of all estimated new cases of cancer in 2020 (2). In 2020, the single leading type of cancer in the United States was breast cancer (11.1%) followed by lung cancer (10%), prostrate (9,2%), colorectum (6.8%), and melanoma of the skin (4.2%). Despite the significant prevalence of cancer and numerous clinical trials conducted for oncology treatments, data have shown an almost 95% attrition rate for anticancer drugs from Phase I trials until marketing authorization. Various factors such as inaccurate preclinical models, lack of suitable biomarkers in clinical trials, and a disconnect between industry, academia, and regulators are responsible for the high attrition rate (3). Therefore, it is vital to develop suitable study designs and protocols for candidate molecules such that they obtain regulatory approval and can be marketed. In addition to these challenges, the development of anti-cancer agents comes at a monumental cost of an estimated $2.8 billion. Several factors such as choice of relevant endpoints, choice of appropriate biomarkers that are guided by tumor biology, and careful patient selection are expected to improve the overall fate of oncologic agents in the clinical trial phase.
The United States Food and Drug Administration (FDA) has released several guidance documents over the years through the Oncology Center of Excellence to support the development of oncologic treatments and diagnoses. Furthermore, information on the clinical trials for the treatment of different types of cancer or specific interventions can be found on the National Cancer Institute (NCI) website and ClinicalTrials.gov. Currently, ClinicalTrials.gov, a website maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH) contains listings of publicly and privately sponsored trials and includes information on 91,937 studies related to cancer indicating the high volume of research being conducted in this field (4).
How do oncology clinical trials differ from clinical trials for other diseases or conditions?
In contrast to clinical trials for other diseases and conditions, oncology clinical trials are generally more complex and are not necessarily focused on only safety and efficacy. The following are some of the points that differentiate cancer clinical trials from those for other conditions:
- The complex nature of cancer includes both the type of cancer affecting different organs as well as different cancer subtypes that involve tumor development in different ways. This makes cancer not a single disease but several different diseases that can complicate drug development.
- Most trials are focused on safety and efficacy endpoints with measurable outcomes of morbidity and mortality. However, most cancer trials are focused on outcomes such as improvement in quality of life and reduction in adverse effects that are especially relevant for chemotherapeutic agents.
- The type of comparator treatment in cancer trials is always the standard of care in contrast to placebo arms that are commonly used in trials for other conditions. It is essential not to interrupt a cancer patients’ regular medication schedule as this can lead to life-threatening complications.
- Adverse events in cancer trials are scored according to guidelines provided by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) from Grade 1 to Grade 5 with increasing order of severity. For most other treatments, adverse events are bracketed into three categories: mild, moderate, or severe.
- Patient recruitment is a significant challenge in cancer trials especially in the current era of precision medicine as it is difficult to find patient subgroups with certain genetic profiles. Patient recruitment is challenging and poses a significant financial burden on sponsors and a lack of understanding and access to travel sites is also a hurdle in the recruitment process.
- Choice of trial design should consider several factors such as the cancer type and subtype, endpoint, and adverse effects. This necessitates the development of a well-thought-out design that addresses all the questions in a statistically robust manner while still being flexible enough to handle recruitment challenges that can occur.
Types of cancer clinical trials
Cancer clinical trials are of different types depending upon the objective which can be treatment, prevention, screening, improvement of quality of life, or natural history studies. Studies have shown that early diagnosis of cancer can help slow or halt its progression and recent research has linked molecular or genetic factors to cancer development. Therefore, it is necessary to develop not only interventional trials but also those for diagnosis. Also since cancer is a chronic condition it is necessary to develop treatments that can improve the patient’s quality of life. The various types of cancer trials and the questions they seek to answer include (5):
Treatment trials:
Compare existing treatments or standards of care to new treatments and are conducted on cancer patients. The interventions include drugs, vaccines, immunotherapy, and surgical or radiation approaches and seek to answer questions related to safety, efficacy, dosage form, dose regimen, and side effects.
Prevention trials:
Are conducted on healthy volunteers or cancer-free participants who have a high risk of developing the disease based on genetic or environmental factors. These trials can be ‘action studies’ that involve studying the effects of exercise or diet on cancer prevention or ‘agent studies’ that involve determining the effects of dietary supplements and drugs on cancer incidence. The safety and ability of these methods to prevent cancer are studied by investigators.
Screening trials:
These involve carrying out tests or diagnostic procedures that can help in the early detection of cancer thereby lowering deaths caused by the disease. Investigators compare various screening studies and study the link between cancer diagnosis and progression to screening study.
Quality-of-life or Palliative Care trials:
They are conducted on cancer patients with the aim of finding ways for patients to cope with the disease or side effects of treatment. These trials involve testing drugs or studying other ways to deal with the disease such as exercise, attending support groups, or counseling (psychosocial interventions).
Natural history studies:
These prospective long-term studies are conducted on cancer patients or those with are predisposed to cancer risk based on genetic factors and involve the collection of family details and samples to answer various questions related to cancer development.
Study designs for cancer clinical trials
Depending upon the objective, the study design for cancer clinical studies falls into two main categories: observational studies and experimental studies. Observational studies or epidemiological studies involve studying subjects in natural settings or comparing already administered interventions that are not under the control of the investigator. In contrast, experimental studies involve the administration and comparison of interventions to participants in a controlled setting by the investigator in a randomized manner. Although experimental studies are more reliable and unbiased, they are often difficult to conduct and expensive. Observational studies provide information about long-term outcomes and real-world usage and a lot of descriptive data can be obtained from them. Therefore, the choice of the type of study depends upon factors such as funding, availability of data, participant recruitment, and time. Figure 1 shows the different types of study designs for each of these study types.
Figure 1. Study designs for observational and experimental cancer clinical trials
Observational studies:
- Case-control study: these are retrospective studies that involve the comparison of individuals with cancer (cases) to those without cancer (controls) and develop a causal relationship between the disease with some exposure such as genetic or lifestyle differences.
- Cohort study: these prospective studies involve observation of participants that have common characteristics over a long time to identify which of them develop a disease, for example, cancer based on exposure to certain factors. They can help determine whether certain nutrients, diet, exercise, or drug treatments can help prevent or cause cancer.
- Case report and case series: these studies involve descriptive reports of a single patient or a group of patients with similar diagnoses that are used for the study of rare diseases as they contain comprehensive information about new/unusual conditions. They contain demographic information such as age, gender, ethnicity, and co-morbidities and are useful for obtaining information on the efficacy of new interventions and can help guide further research.
- Cross-sectional study: these studies occur at a single point in time and can be used to determine if exposure to certain factors causes the outcome of interest in a particular population. In cancer, cross-sectional studies may be used to determine if administration of a certain drug treatment reduces the prevalence of cancer after maybe 5 years of treatment
Experimental studies:
Most interventional or experimental trials are randomized controlled trials (RCTs) which means that a homogeneous group of participants who are selected based on eligibility criteria are randomly divided into two groups. Successful randomization ensures that the two groups are similar in confounding factors which reduces or eliminates the chances of bias. One of the groups receives the intervention and the other is the control group (placebo or standard treatment). Any difference in outcomes between the groups is attributed only to the intervention
- Double-blind randomized trial: in this type of RCT, neither the participants nor the investigators/researchers are aware of treatment assignment which greatly reduces the chances of performance bias.
- Single-blind randomized trial: in this design, only the participants are unaware of their treatment.
- Open or unblinded trial: in this type of design, both the researchers and participants are aware of their treatment and is used when blinding is not possible such as surgical interventions.
- Non-randomized trials: the intervention and control treatments are assigned to two groups without any randomization protocol thus leading to bias in the results.
Advancements in oncology clinical trial designs
Current and progressing research in the field of genomics and big data has led to the development of precision medicine in oncology research. Precision or personalized medicine involves the identification of disease targets based on their genetic make-up for the development of targeted therapies against them (6). In the United States, the ‘All of Us’ initiative developed by the NIH aims to create a database of demographic and biological data from at least a million individuals to determine the influence of biology, lifestyle, and environmental factors on overall health. This information is expected to be used for precision care in oncology and other fields (7). Tumor proliferation and survival are often related to a single oncogene which causes the production of mutant proteins that can serve as the target for therapies leading to the concept of biomarkers. Biomarker identification is the key for detection and estimating the risk of cancer as well as developing targeted therapies. The US FDA has released guidance on the design strategies to expedite the development of oncology drugs which include basket and umbrella trial designs (Figure 2).
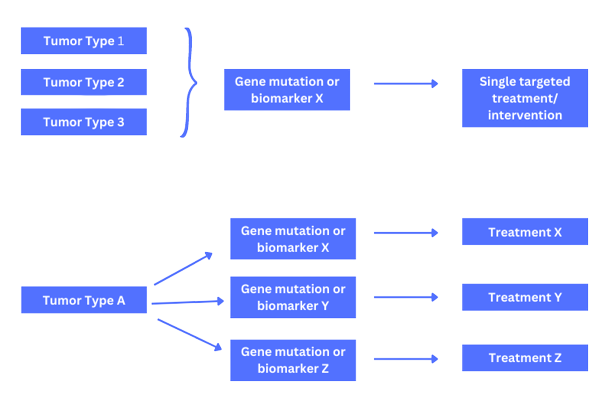 Figure 2. Basket and Umbrella trial designs
Figure 2. Basket and Umbrella trial designs
- Basket trials test the effect of a single targeted drug treatment or combination on patients with different types of cancer that share similar molecular alterations. The patients included in these trials may have cancers affecting different parts of their body but all with the same genetic mutation. An example of a basket trial was the Vemurafenib trial in multiple non-melanoma cancers with BRAF V600 mutations.
- Umbrella trials involve the evaluation of the effect of multiple drugs simultaneously in patients with the same type of cancer but with different genetic mutations in each tumor. The plasmaMATCH was an umbrella trial that evaluated 5 different therapies for advanced breast cancer based on different molecular signatures.
Adaptive trials
As noted earlier the success rate of oncologic agents is disproportionate to the prevalence of cancer and the high attrition rate can be attributed to inappropriate study designs and failure of potential treatments in Phase I trials owing to the inability to identify suitable patient populations. Also, failure of drugs to reach patients is associated with tremendous costs and resources. Adaptive trial designs present a novel way to overcome the problems associated with traditional clinical trial models that are based on the design, conduct, and analyze paradigm. Some of the features of adaptive trials include changes to be implemented as the trial progresses. These changes could include:
- Adjustments in sample size
- Modifications of dose and dosing schedules
- Changes in treatment-arm allocations
- Focus on patients that are likely to benefit from the treatment
- Early stopping decisions
In 2019, the US FDA has released a draft guidance document on adaptive trial designs where the importance of preplanned modifications was emphasized (8). In precision oncology, adaptive trials are particularly useful during early-phase studies where appropriate patient populations can be chosen based on biomarkers responses, and in basket and umbrella trials as well as the maximum tolerated dose (MTD) or dose-escalation studies.
Although conventional observational and interventional study designs are still used to a large extent in oncology trials, the era of genomics is opening avenues to the sophisticated precision oncology field that warrants the use of specialized study designs. However, despite the progress already made, hurdles such as biomarker development and validation and challenges associated with data monitoring remain and need to be addressed to allow for the availability of targeted cancer treatments with better treatment outcomes.
References
- Cancer (who.int)
- Cancer Today (iarc.fr)
- Full article: How can attrition rates be reduced in cancer drug discovery? (tandfonline.com)
- ClinicalTrials.gov
- Types of Cancer Clinical Trials – NCI
- An overview of precision oncology basket and umbrella trials for clinicians – PubMed (nih.gov)
- All of Us Research Program | National Institutes of Health (NIH)
- Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry | FDA






