In India, the Central Drugs Standard Control Organization (CDSCO) under the Directorate General of Health Services, Government of India is the National Regulatory Authority (NRA) responsible for the approval of drugs, the conduct of clinical trials, setting standards for drugs, and regulating standards for imported drugs under the Drugs and Cosmetics Act, 1940 and rules, 1945. In 2016, the CDSCO introduced an online portal called SUGAM which is an e-Governance system used to discharge various functions of the CDSCO. This online platform enables applicants to obtain licenses, permissions, approvals, and certificates along with tracking and downloading features. It is necessary for applicants to register on the portal and be approved with an online account to avail of the services. Sponsors of clinical trials and bioavailability and bioequivalence (BA/BE) studies, importers, ethics committees, and research and development (R & D) organizations can register on SUGAM. Various forms are available for different functions such as:
- Form CT-04: grant of permission to conduct a clinical trial of a new drug or investigational new drug (IND)
- Form CT-05: grant of permission to conduct a BA/BE study in human subjects
- Form CT-16: Import of drugs for clinical trials
The SUGAM online portal offers advantages such as:
- Ability to easily track the status of submissions
- Respond to queries raised in a timely manner
- Convenience of uploading necessary documents
- Direct application of import licenses to the CDSCO
- Transparency in the review and submission process
- Notification and time tracker section for a response from CDSCO
Clinical Trial Application Requirements
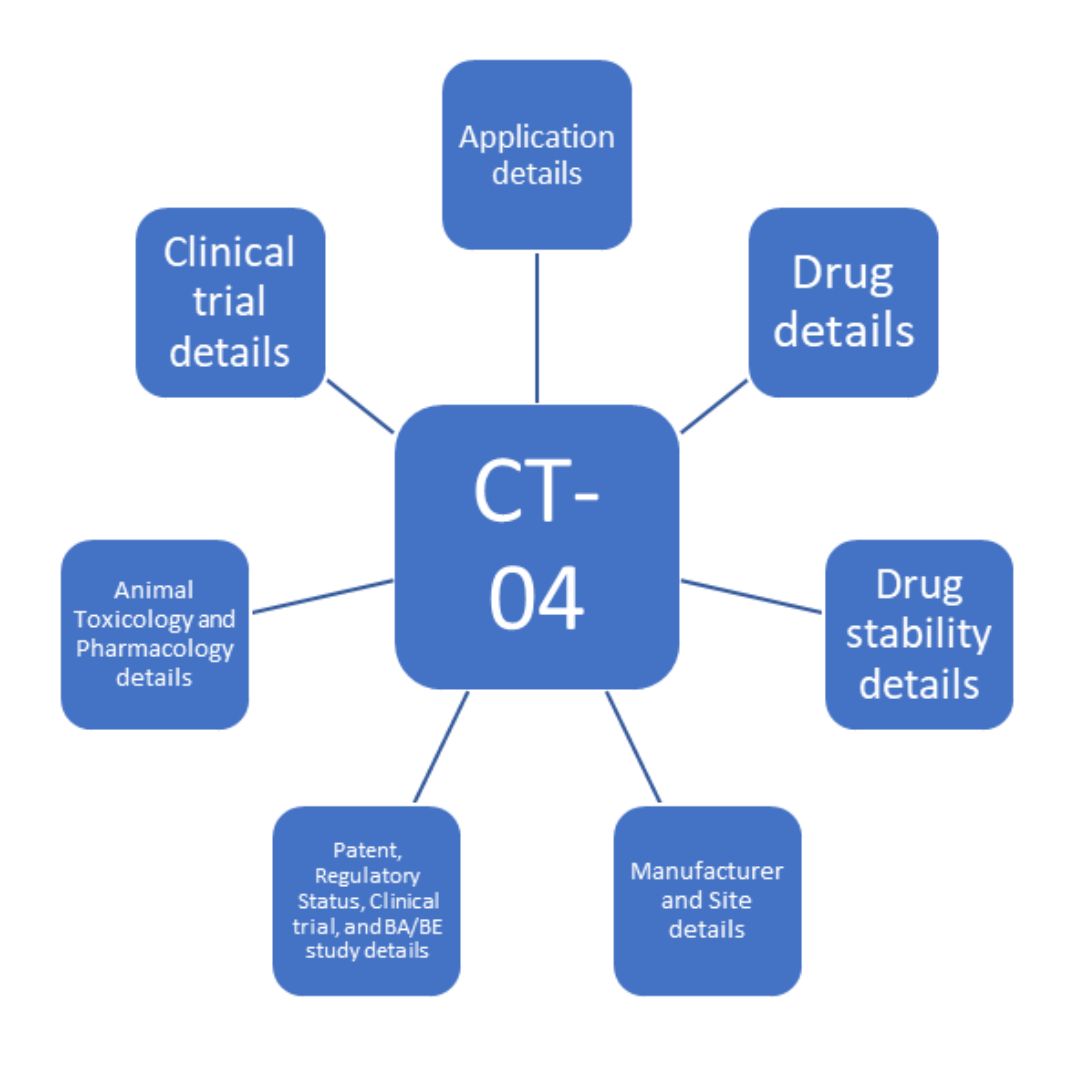
Online Form CT-04 contains the information that is necessary to fill in an application for clinical trial conduct (Phase 1/Phase 2/Phase 3/Phase 4). Figure 1 shows the broad areas that are required to be populated for either new drugs, subsequent new drug (SND), or fixed dose combinations for grant of permission to conduct any phase of a clinical trial.
Figure 1. Overview of information required on SUGAM portal for clinical trial application
Under each of these sub-headings, further information is required as follows:
Application details
- Purpose of application: manufacture, import, undertake a clinical trial
- Category: new drug, bulk drug, finished formulation
- Type of drug: single ingredient or FDC
Drug details
- Generic name
- Dosage form
- Indication
- Route of administration
- Pharmacological classification
- Pack presentation: primary packaging material, dose, pack size
- Storage condition: temperature, humidity, light, proposed shelf life
- List of ingredients: name, strength, label claim, manufacturer name
- List of impurities
Drug stability details
- Batch details: batch number, batch size, presentation
- Duration of stability study
- Stability condition: temperature, RH
- Storage condition
- Assay: parameter, specification, result
Manufacturer and Site details
- Site type
- Manufacturing unit
Patent, Regulatory Status, Clinical Trial, and BA/BE Study Details
- Patent details in India
- Clinical Trial and BA/BE Study status
- Regulatory Status of the Investigational Product in other countries
Animal Toxicology and Pharmacology Details
- Systemic toxicity studies: single dose, repeated dose, male fertility, female reproduction, and developmental toxicity studies
- Local toxicity studies: dermal, ocular, inhalation, vaginal, photo allergy, rectal tolerance test
- Genotoxicity
- Allergenicity/Hypersensitivity
- Carcinogenicity
- Specific Pharmacological Actions
- General Pharmacological Actions
- Safety Pharmacology Studies
- Pharmacokinetic studies: absorption, distribution, metabolism, excretion
- Training of safety Pharmacology Studies
- Application of Good Laboratory Practices (GLP)
Clinical data
- Scope/Objective of the trial
- Study design
- Global clinical trial
- Sponsor details: address, contact details
- Comparator drug details: name, dosage form, route of administration, name of company, and country
- Disease name
- Clinical trial site details: address, ethics committee
- Trial-related documents: study protocol, patient information sheet, informed consent form (ICF), financials of applicant, case report form 9CRF) template, investigator’s brochure, list of participating sites, investigator’s undertaking, ethics committee approvals, draft of investigational medical product (IMP) label, insurance certificate, details of central laboratory, risk/benefit assessment, unmet medical need in the country, the rationale for conducting the study in India
Form CT-04 with the above information (specified in the Second Schedule) and fees as per the Sixth Schedule of Clinical Trial Rules, 2019 need to be uploaded and submitted on the SUGAM portal.
In addition to Form CT-04 which contains the information required for the import of drugs, Form CT-05 along with the documents specified in the Fourth Schedule and fees in the Sixth Schedule are needed to be submitted for the import and registration of drugs:
The subsequent details need to be entered in Form CT-05:
Applicant and Manufacturer details
- Type of application
- Applicant details: address and contact details
- Manufacturer details: registered address, contact information
Drug details
- Drug category: bulk drug or finished formulation
- Generic name
- Pharmacopoeialmonograph
- Class of drug
- Shelf life
Manufacturer site details
- Site type
- Manufacturing site address
Required documents
- Cover letter
- Power of attorney
- Copy of import permission
- Copy of wholesale license
- Authorization letter: Indian agents, foreign manufacturers, company
- Site Master File: major equipment in quality control and production, key personnel list, organizational chart, quality assurance functional chart, plant layout, system drawings (HVAC, water, pressure differential, personal movement, material movement), distribution, complaints, product recall SOPs, list of contract manufacturing/analysis
- Drug Master File: process flow chart, manufacturing process development report, control of critical steps, process validation report, list of byproducts, impurities, related substances, residual solvents, and their testing procedures, structure elucidation of impurities and toxicity report, details of input materials with specification and sources, source and maintenance of reference standards, container-closure system and testing procedure, batch determination, specification and justification of products used in manufacturing, certification of analysis, material safety data sheets (MSDS)
- Stability data: long-term, accelerated data and summary reports
- Certificates: manufacturing license/product authorization certificate, GMP certificate, COPP certificate, FSC certificate
- Product registration certificate, certificate of suitability from EQDM, original label/specimen label, quality statement, statements of change in manufacturing process/packaging/labeling/testing/documentation, and change in the constitution/address of the firm total quantity exported to India in 3 years
Data required for CT-16 application for grant of license to import new drug or investigational new drug for clinical trial or bioavailability or bioequivalence study or for examination, test, and analysis:
- Name of applicant
- Nature and constitution of the applicant
- Corporate or registered office address and applicant address including telephone number, mobile number, fax number, and e-mail id
- Details of new drugs to be imported
- Particulars of overseas Manufacturers, Manufacturing sites
- Fee paid
- Intended use declaration letter
- Annexure1:
- Details of a new drug or investigational new drug:
- Names of the new drug or investigational new drug:
- Therapeutic class
- Dosage form
- Composition
- Indications
- Details of manufacturer and manufacturing site
- Name and address of the manufacturer
- Name and address of manufacturing site
The CDSCO’s SUGAM portal serves as a convenient way to submit applications and obtain permissions for clinical trials, manufacture, and import of drugs. It enables applicants to track their application status and have a ready checklist of documents necessary for various permissions. It is necessary for manufacturers and importers to be familiar with this system to facilitate their regulatory work as it provides an integrated workflow all the way from application to grant of licenses.
References: