Biostatistics involves the application of statistical techniques for the design and analysis of clinical trial data. In addition to the conduct of trials which encompasses regulatory approval processes, informed consent, and patient monitoring, and safety, it is necessary to ensure that trials are appropriately designed to have suitable statistical power to meet the end point(s) and the correct trial design is employed to prove the hypothesis. As important as any of these is that the data gathered is analyzed and interpreted appropriately as it is used for clinical decision-making and that the data is collected in such a way that makes it reliable, valid, and accurate. Biostatistics involves processes such as the detection of patterns in data sets (descriptive statistics), statistical tests applied to data to make conclusions regarding populations (inferential statistics), and statistical modelling techniques to determine relationships between factors.
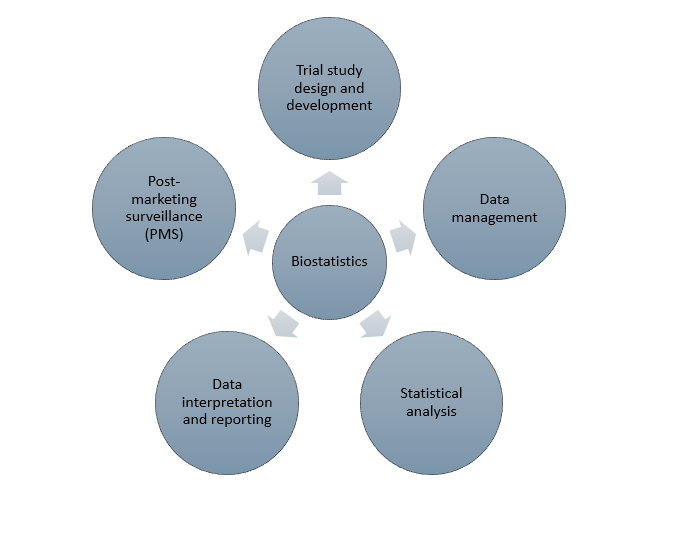
Biostatistical services form an important aspect of all steps in a clinical trial from design, data collection, analysis, interpretation and reporting, and post-marketing surveillance (PMS). The figure below shows the various steps in which biostatistics is used in clinical trials.
Trial design and development
Before the inception of a clinical trial, it is necessary to have a robust and comprehensive statistical analysis plan (SAP) in place that provides a detailed description of the various statistical tests and analysis methods that will be used to analyze the collected qualitative and quantitative data. SAPs are required to be submitted along with the study protocol prior to initiation of a clinical trial to regulatory agencies. SAPs contain the following information:
- primary and secondary end points
- details for interim analysis
- handling of missing data
- sample size calculations
- final analysis details
- significance levels
- statistical tests intended to be used
- tables and figures to be included in the analysis reports
- randomization plans, etc.
- trial design (superiority, equivalence, non-inferiority, adaptive, basket, umbrella)
Data management
Biostatistics forms an integral part of the data collection process wherein it is necessary for biostatisticians to be involved in various activities related to data management such as:
- the design of data collections instruments such as case report forms (CRFs) and electronic databases if they are used to capture patient information
- validation of instruments such as questionnaires and patient-reported outcome (PRO) instruments
- statistical methods to ensure the accuracy, completeness, and reliability if the data and conduct interim analysis if needed to help adjust the protocol.
Validity of data is critical for regulatory approval making it imperative to ensure the correctness of data entry taking care to avoid errors and inconsistencies. Biostatisticians must help design CRF and analyzable data sets as per Clinical Data Acquisition Standards Harmonization (CDASH) and Clinical Data Interchange Standards Consortium (CDISC) standards.
Statistical analysis
Often the most important role of biostatistics in clinical trials is the statistical analysis component. This involves the use of various statistical analytical techniques such as
- inferential statistics
- descriptive statistics
- multivariate and linear regression models (to draw relationships between two variables in the population)
- survival analysis (Kaplan-Meier and Cox proportional hazards model)
Data interpretation and reporting
Following analysis of datasets from sample patient groups, it is necessary for biostatisticians to be involves in the interpretation of data such that can be put into an easily readable format that can be understood and applied by clinicians and other healthcare professionals (HCPs). Cross-functional organization and communication between biostatisticians and clinical teams is necessary to help in interpretation of results and manuscript preparation activities such as the inclusion of key figures and tables to represent important findings. Submission to most regulatory agencies requires the involvement of biostatisticians to create statistical analysis reports or clinical study reports (CSR) that present the statistical data in certain formats.
Post-marketing surveillance (PMS)
Phase IV trials or PMS studies are becoming increasingly important to inform healthcare decisions from data that is gathered in the real-world. This data is often inconsistent as it is collected from disparate sources and of large volume making it essential to have a biostatistics team in place to handle this data and enable valid conclusions to be drawn from it. Biostatistics is applied in real-world evidence (RWE) analysis which involves analysis of data from real-world sources such as insurance claims, patient registries, and electronic health records (EHRs) to help identify relationships between variables. Potential safety signals can also be detected using biostatistical methods that form a part of adverse event reporting and pharmacovigilance studies. Meta-analysis, survival analysis, and risk evaluation and mitigation strategies (REMS) also involves the use of biostatistics in PMS.
Biostatistics is an integral part of all steps of clinical trials encompassing trial design to study publication. It helps ensure that the data collected is accurate and reliable and valid conclusions are drawn from the data that feed into further decision-making.
Read More: https://prorelixresearch.com/cdisc-standards-for-biostatistics-and-clinical-data-management/
References
- Understanding Biostatistics Interpretation – StatPearls – NCBI Bookshelf (nih.gov)
- Biostatistics: A toolkit for exploration, validation and interpretation of clinical data – PMC (nih.gov)
- Guidance for biostatisticians on their essential contributions to clinical and translational research protocol review – PMC (nih.gov)