FDA Validation Rules for Submission Data are essential for regulatory compliance. Learn about FDA data submission requirements, validator rules, study data standardization plans, and Pinnacle 21 validation. Stay updated with the latest FDA Technical Conformance Guide 2024 to avoid costly mistakes and streamline submissions.
Validation Guidelines For Submission Data
Compliance with rigorous policies and proper preparation greatly influence the submission of study data to the FDA. The validation rules for submission data guarantee submission validation for dataset integrity and accuracy, coherency, and submission formatting through dataset standardization policies and procedural guidelines.
The ALCOA+ principles provide a fundamental framework for data integrity in clinical trials, ensuring data quality and reliability. ALCOA emphasizes that data must be Attributable, Legible, Contemporaneous, Original, and Accurate. The “+” expands upon these, requiring data to be Complete, Consistent, Enduring, and Available. These principles, integral to Good Clinical Practice (GCP) guidelines, are validated to guarantee adherence to these high data integrity standards. Not complying with set standards will prove very costly due to application rejection, significant financial investment into redoing forms of payment, and submissions because of extensive resubmission work that needs to be done.
Key FDA Data Submission Requirements
Submission of the data framed within preset boundaries described by FDA data submission requirements entails submission of data files that must comply with regulatory criteria at the specified timeframe. The FDA requires sponsors to submit clinical and non-clinical study data in standardized formats, such as the Study Data Tabulation Model (SDTM) and Standard for Exchange of Nonclinical Data (SEND). These formats allow ease of use during reviews and submissions to regulatory bodies.
Importance of the FDA Data Standards Catalog
As a guide for implementation for sponsors to ensure their data achieves submission to the FDA expectations, the Directory of FDA Standards Catalog emphasizes boundless data terminologies. Its description, accepted forms of expression, and constant updates accompanying changes, modification to intricate sponsor industry standards, assures data substantiation.
Utilizing FDA Validator Rules for Compliance
Prior to review by the relevant authorities, automated examinations that guarantee the necessary requirements have been achieved for each piece of submission data, coherence, and cross-reference quietness check formatting guides through FDA validator rules.
These guidelines uncover mistakes, discrepancies, and potential formatting issues that could impede the timeline of approval. Software such as Pinnacle 21 offers assistance to sponsors by checking their datasets against the FDA’s guidelines.
Formulating a Compliant Study Data Standardization Plan for the FDA
The blueprint of a Study Data Standardization Plan (SDSP) FDA delineates actions that sponsors intend to take with study data organization and submission. The SDSP attempts to circumvent potential problems of non-conformity by ensuring compliance with FDA regulations.
A well-crafted SDSP increases the likelihood of an efficient regulatory review instead of a high chance of rejection in submissions.
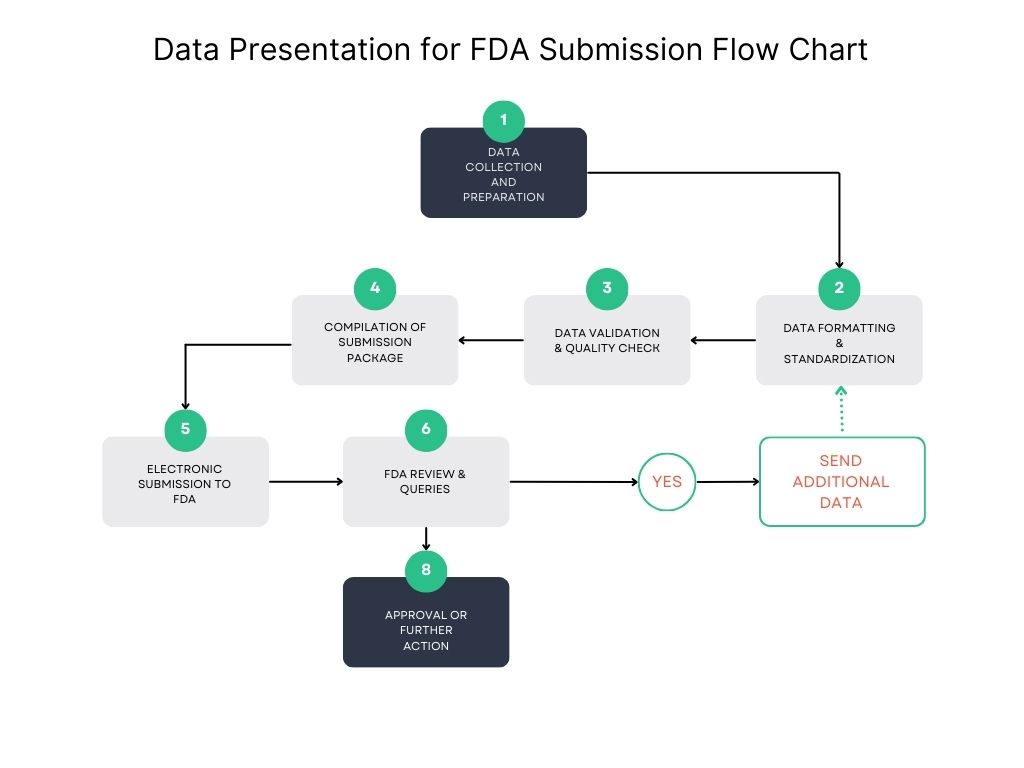
Data Presentation for FDA Submission Flow Chart
An orderly and accurate data presentation for FDA submission for surgical procedures is essential for acceptance from the regulators. The sponsors have to follow a specific order, which makes the data poster easier to read and quicker to review. Along with this data presentation for FDA submission flow chart can serve as a guide in presenting and organizing data logically and plainly.
Key Components of the Data Presentation Flow Chart:
Study Design & Protocol Development
- Define objectives, study endpoints, and methodology.
- Ensure compliance with FDA regulations (21 CFR, GCP).
- Submit the Investigation New Drug (IND) or Investigation Device Exemption (IDE), if applicable.
Data Collection & Validation
- Gather clinical trial or pre-clinical study data systematically.
- Maintain compliance with Good Clinical Practice (GCP) guidelines.
- Validate data accuracy through quality control processes.
Statistical Analysis & Interpretation
- Apply appropriate statistical methods to interpret results.
- Ensure data integrity and reproducibility of findings.
Data Formatting & Structuring
- Present data in tables, charts, and graphical formats as per FDA guidelines. Organize information according to the Common Technical Document (CTD) format, if required.
Compilation of Regulatory Submission Package
- Include summaries, safety reports, and clinical findings.
- Ensure completeness of documents, including case report forms (CRFs) and investigator reports.
Electronic Submission (eCTD format)
- Convert documents into an electronic Common Technical Document (eCTD) format.
- Follow FDA’s electronic submission guidelines.
FDA Review & Response Handling
- Address FDA queries promptly.
- Provide additional data or clarifications as required.
By following this structured Data Presentation for FDA Submission Flow Chart, sponsors can ensure a smooth regulatory review process, minimizing delays and increasing the likelihood of approval.
Understanding FDA-Specific SEND Validation Rules
FDA-specific SEND validation rules are applicable to the submission of non-clinical study data. The rules aim to maintain uniformity, aptness, and compliance concerning Federal standards. Without following these validation rules, sponsors run a high risk of numerous refusals, leading to re-submission and review cycles.
Integrating Pinnacle 21 in Submission Data Validation for FDA Rules
Submission data Pinnacle 21 validation rules for the FDA enables the validation of datasets by the sponsors concerning the preset requirements for regulations.
Pinnacle 21 is a widely used tool in the industry for automating validation, error detection, and compliance checks according to FDA submission guidelines.
FDA Validation Rules for Submission Data SDTM
The submission SDTM validation rules examine compliance in clinical study data and its formatting. The SDTM model standardizes datasets to make the review and interpretation easier.
The submission Study Data Tabulation Model (SDTM) validation rules ensure that clinical study data comply with regulatory requirements and follow a standardized format. These rules help maintain data consistency, accuracy, and reliability, facilitating efficient review and interpretation by the U.S. Food and Drug Administration (FDA).
Key Aspects of SDTM Validation Rules:
Conformance to SDTM Standards
- Data must align with the CDISC SDTM Implementation Guide (IG), ensuring correct domain structures, variable names, controlled terminology, and metadata.
Dataset Structure and Format
- Each dataset should follow the prescribed row and column structure, maintaining the integrity of clinical trial data. Required, expected, and permissible variables must be correctly included.
Consistency Across Datasets
- Relationships between datasets should be maintained (e.g., linking demographics (DM) with other subject-level data).
- Key variables such as USUBJID (Unique Subject Identifier) must be consistent across domains.
Controlled Terminology Compliance
- Values must conform to CDISC Controlled Terminology to ensure uniformity and minimize ambiguity in clinical data interpretation.
Referential Integrity
- Values in related datasets must match (e.g., AE (Adverse Events) dataset should reference valid subjects from the DM dataset).
Metadata and Define.xml Compliance
- The Define.xml file must accurately describe dataset structures, variables, and controlled terminology.
- It should provide clear documentation to support data traceability.
Business and Regulatory Rules Compliance
- Ensuring adherence to regulatory expectations, such as FDA Study Data Technical Conformance Guide (TCG) and eCTD submission guidelines.
- Common issues include missing values, incorrect data types, and mismatches between datasets.
Use of Validation Tools
- Sponsors must validate datasets using FDA’s Pinnacle 21 (P21) or other tools to check compliance with SDTM standards.
- FDA’s DataFit Tool helps assess data quality and submission readiness.
Following FDA SDTM validation rules ensures a smooth submission process, reducing delays in regulatory review. Properly formatted and validated datasets enhance data transparency, making clinical trial results easier to interpret and assess for regulatory approval.
FDA Validation Rules for Submission Data Pinnacle 21
Utilizing the FDA Validation Rules for Submission Data Pinnacle 21 allows the sponsors to check their datasets for inconsistencies before submission. Pinnacle’s automated validation checks mitigate the chances of a negative regulatory response or rejection.
Navigating the FDA Technical Conformance Guide 2024
FDA Technical Conformance Guide 2024 has been updated regarding submission of data regulations and expectations. This guide is a must for every sponsor who wishes their data to be in line with the current FDA requirements.
Compliance with FDA Validation Rules for Submission Data is critical for successful regulatory submissions. Sponsors must follow FDA data submission requirements, adhere to the FDA Data Standards Catalog, and utilize tools like Pinnacle 21 to ensure data accuracy. Implementing a well-structured Study Data Standardization Plan, following the FDA Technical Conformance Guide 2024, will help avoid costly mistakes and streamline the submission process. Staying informed about regulatory updates and leveraging validation tools ensures compliance and accelerates regulatory approvals.
Read More: