Clinical trials are necessary in advancing medical research and health care options by allowing new treatments to be introduced in the market through safety and efficacy testing in humans. Successful completion of drug synthesis and combinatorial chemistry/high-throughput screening approaches culminates in a drug candidate or medical device being tested in first-in-human trials. This candidate then progresses through various phases of clinical trials and this ends in regulatory approval or rejection of the candidate based on information gathered from the clinical trial. The United States Food and Drug Administration (FDA) defines a clinical trial as a research study that involves human subjects and a test article and is subject to FDA submission requirements. There are various reasons for conducting trials such as determining the safety and efficacy of new treatments versus existing treatments or standard of care, expanded label access of the treatment to different conditions and populations (paediatrics), and developing new diagnostic methods.
Clinical trials are voluntary and are conducted as per the following phases:
Phase 1
In this phase, 20-100 healthy volunteers or patients with the disease/condition are chosen and administered the test article or active comparator/placebo for a period of several months to gather information about the safety and dosage of the test article.
Phase 2
Several hundred patients with the disease/condition are administered the test article or placebo/active comparator depending upon the study design in this phase to collect information regarding efficacy and side effects of the test article.
Phase 3
The treatment benefit of the test article is tested in 300-3000 patients with the disease/condition and safety data is collected over a longer duration of 1-4 years in this phase.
Phase 4
This phase is conducted post-marketing approval of the test article and helps capture data from several thousands of patients with the disease/condition that are using the test article. Mainly done to obtain information about side effects that may occur after a longer time and rare events that are not captured during the clinical trial phases.
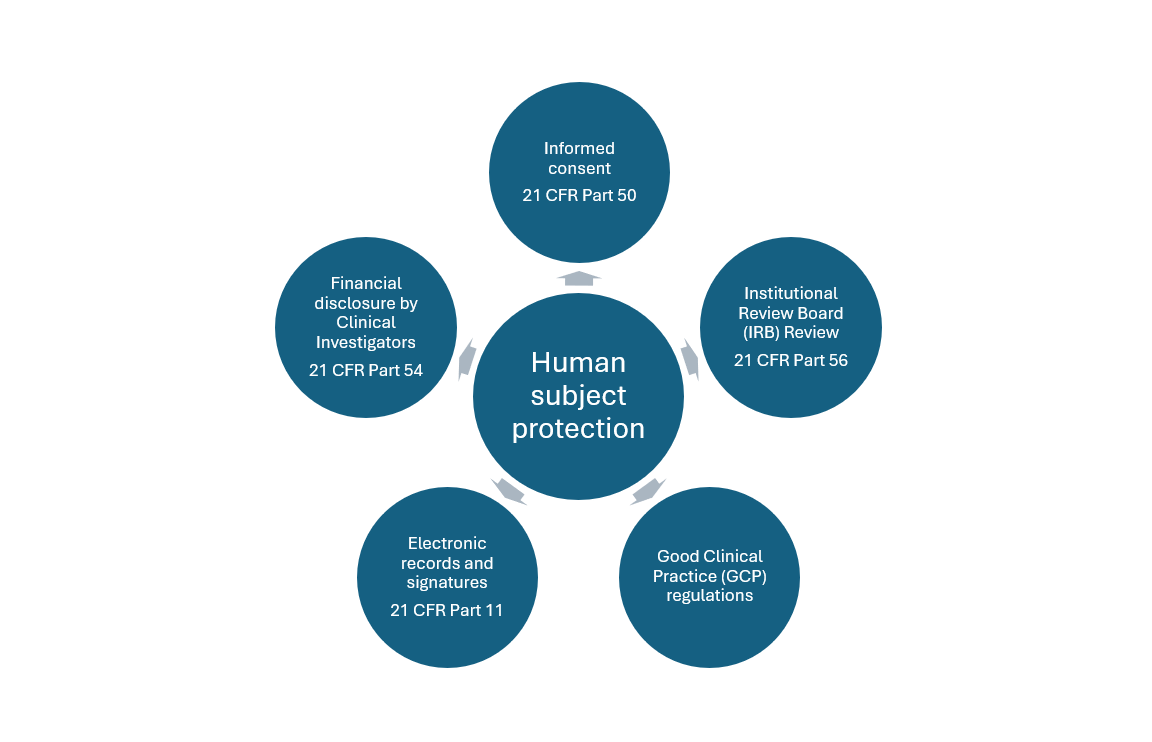
The purview of the FDA involves ensuring that trials are designed and conducted in a scientifically sound manner and that the sites and personnel involved in trial conduct are qualified. Adherence to Good Clinical Practice (GCP) guidelines and other regulatory guidelines is also essential in ensuring that the data gathered during the trial is consistent, accurate, and reliable as health care decision making often hinges on this data. In addition to this, the FDA has developed strict guidelines to protect the rights, health, well-being, safety, and confidentiality of human subjects as part of their mission which are outlined in the Code of Federal Regulations (CFR).
Informed consent (21 CFR Part 50)
Under the regulations of the Food, Drug, and Cosmetic Act, it is necessary that all investigators obtain informed consent for participation in the trial from the subject or their legally authorized representative (LAR). This part further explains that no subject should be coerced into participating in a trial and should be explained the risk-benefit profile of the drug/treatment in an easily understandable language or format that its suitable to them. Furthermore, waivers or exceptions to informed consent taken by the Institutional Review Board (IRB) are allowed only in certain circumstances where there is minimal risk involved to the subjects. It is essential to obtain written informed consent from subjects or their LARs and should be signed and maintained as documentation. This chapter ensures that the rights of patients are protected, and they are not influenced into making any decisions that can jeopardize their well-being.
Institutional Review Board (IRB) (21 CFR Part 56)
IRBs are responsible for reviewing clinical investigations under the FD&C Act to ensure that patient’s safety, rights, and welfare are of paramount importance. They review all documents related to the conduct of trials such as the protocol, investigator’s brochure (IB), and informed consent documents. Evaluation of the progress of the trial and adherence to ethical standards is also the responsibility of the IRB. It is essential for the IRB to have qualified members that are professionally competent, and the IRB should be registered with supporting information. The IRB’s main mission is to ensure that the clinical trial proceeds as anticipated and that any changes to the protocol are approved such that no untoward harm is caused to the participants.
Good Clinical Practice (GCP) regulations
These are international standards that ensure the ethical conduct and reliability of data from clinical trials. The International Conference for Harmonisation (ICH) E6 GCP guidelines are implemented by the FDA through outlining IRB responsibilities, informed consent, investigator and sponsor responsibilities, safety reporting information, audits, quality assurance measures, continuous monitoring, safety and adverse event reporting, and data confidentiality as per Health Insurance Portability and Accountability Act (HIPAA).
Electronic records and electronic signatures (21 CFR Part 11)
This section establishes requirements for electronic records and signatures to ensure the reliability and trustworthiness of electronic documents submitted to the FDA. These are used on informed consent documents and other work submitted to the IRB and ensure patient security and confidentiality. Since these ensure that permission is granted to only authorized personnel, tampering and changes to data are not permitted.
Financial disclosure by clinical investigators (21 CFR Part 54)
This part involves the applicant to disclose financial details and agreements between the sponsor and clinical investigators(s) as these may affect the results of the study and outcomes. This includes details of compensations, equity and proprietary interests and these disclosure statements should be maintained properly and available for scrutiny if required. This part helps to protect the integrity and safety of participants by ensuring that the data collected that leads to the outcome of the study is fair and reliable.
Thus, the FDA has several regulations in place that help protect the safety, welfare, and confidentiality of the participants. These safeguards are critical to the ethical conduct of clinical studies and ensure the reliability of data. This is extremely important as major healthcare decisions are based on the outcomes of clinical trials.
Read More: Food and Dietary Supplements Clinical Trials Regulations in USA
References
- Step 3: Clinical Research | FDA
- FDA Policy for the Protection of Human Subjects | FDA
- ICH_E6(R3)_DraftGuideline_2023_0519.pdf
- eCFR :: 21 CFR Part 54 — Financial Disclosure by Clinical Investigators
- eCFR :: 21 CFR Part 50 — Protection of Human Subjects
- eCFR :: 21 CFR Part 56 — Institutional Review Boards