
Clinical trials are critical for the approval of new drugs and medical devices necessitating that data that is derived from them to make regulatory decisions is done so in a manner that generates accurate and reliable data. Thus, clinical data management is an integral component of all trials from patient recruitment and informed consent to statistical analysis as the approval of new products hinges upon this data. Clinical data management (CDM) includes all processes and activities that involve collection, validation, management, and storage of clinical trial data. To ensure the accuracy, integrity, and completeness of the collected data, there are certain data standards that must be adhered to throughout the trial process. This facilitates sound decision-making by regulatory authorities keeping not only efficacy and safety in mind but also patient well-being, and data security.
As per the United States Food and Drug Administration (FDA), study data standards describe a standard way to exchange clinical and nonclinical data. They provide a consistent general framework for organizing study data, including templates for data sets, standard names for variables, appropriate controlled terminology, and standard ways of analyzing data with common variables. The FDA website has a dedicated resource centre that describes tools and guidances for the submission of clinical data for drugs and biological products including real-world data which can be submitted electronically. It also includes specifications for certain products/conditions and specific studies such as those related to carcinogenicity.
FDA clinical data standards allow exchange of information between sponsors, investigators, and regulatory agencies and ensure accuracy, reliability, consistency, and interoperability. The FDA has adopted Clinical Data Interchange Standards Consortium (CDISC) standards. CDISC is a multidisciplinary, non-profit organization that has developed standards to support acquisition, exchange, submission, and archival of clinical data and metadata to track deletions or modifications to entered data. CDISC has developed global standards for clinical studies and data sharing and includes data collection templates, data formatting tools, and dataset structures that allows for standardized methods for collection, managing, and sharing of clinical data. These templates and standardized data forms ensure reliability of data especially when clinical trials are conducted globally, and several trial sites and coordinators are involved.
The CDM process is composed of several steps such as data form design, data entry and validation, data cleaning, database management, quality assurance, data reconciliation, database lock and archival and storage. In multi-site trials and to allow for exchange of data with regulatory authorities, data transfer, privacy, and security are important aspects of the CDM process.
Figure 1 shows the clinical standards applied by the FDA for different steps of the data management process as per CDISC.
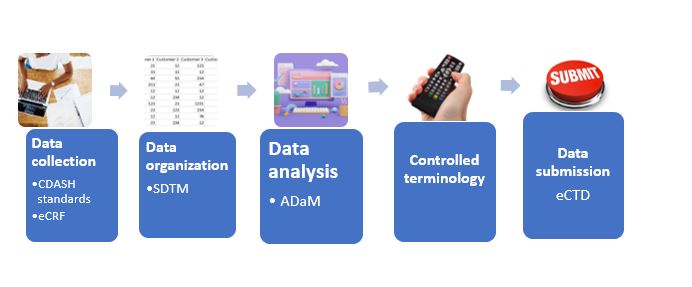
Figure 1. CDISC standards for clinical data management
Clinical Data Acquisition Standards Harmonization (CDASH)
Is CDISC’s standard for data collection and data capture. CDASH provides a standard way to collect data consistently across studies and sponsors that allows for traceability of data and transparency to regulators and integrates with SDTM and ADaM creating consistency throughout the trial lifecycle. It can be used to create case report forms (CRFs) and provides templates for CRFs with standardized data collection fields such as interventions, demographics, medical history, adverse events, and laboratory tests. CDASH is compatible with a variety of electronic data capture (EDC) systems and utilizes standardized terminology and coding to describe medical issues thereby removing ambiguity in the collection process. The goal of CDASH is to ensure collection of high-quality and standardized data.
Study Data Tabulation Model (SDTM)
Provides a standard for organizing and formatting data to streamline processes in collection, management, analysis, and reporting. This CDISC standard organizes data into various domains such as demographics, adverse events, laboratory tests, concomitant medications, vital signs, etc. for submission to regulatory agencies and is used widely by global regulatory agencies and academic centres. It is necessary for raw data to be transformed to and validated to SDTM-compliant domains using mapping specifications to minimize inconsistencies and errors. SDTM makes data sharing and analysis easier as data is standardized.
Analysis Data Model (ADaM)
Is a set of dataset and metadata standards and guidelines developed by CDISC that describes the preparation and organization of analysis datasets for clinical trials. It allows for efficient generation, replication, and review of clinical trial statistical analyses and traceability. The main purpose of ADaM is to provide standardized structures for datasets that allows for analysis and speeds up submission and regulatory processes. The datasets include domains and variables that should be included in the datasets and uses CDISC standardized terminology and describes guidelines for the use of statistical analysis software like SAS.
Controlled terminology
It is the set of codelists and valid values used with data items within CDISC-defined datasets and provides guidance on submission of data items in electronic datasets. It provides a standardized way to describe and document data allowing for uniformity across different data collection platforms. The controlled terminology is organized into domains which contains items and terms with specific definitions and synonymous terms along with data type and format on how the data should be reported. The overall aim of controlled terminology is to ‘speak a common language’ and allow for crosstalk between studies and is necessary for regulatory compliance.
Electronic Common Technical Document (eCTD)
Facilitates submission of nonclinical, clinical, and manufacturing information to regulatory agencies using a structured format. It helps streamline submissions allowing for easier review and processing by regulatory agencies and this format is mandatory for submissions to the FDA. Data that is organized as per SDTM and ADaM standards can be included in eCTD submissions.
Adherence to clinical data standards is important for regulatory submissions and helps ensure accuracy, consistency, interoperability, and traceability of data. This is particularly important today as global clinical trials are gaining popularity and most data is entered electronically for data sharing that is amenable to standardization. CDISC standards can be integrated with each other and ensure high data quality throughout clinical trials and are used by the FDA.
Read More:
References




