
The rising prevalence and large market share of biological products emphasize their importance as treatment options for several cancers and autoimmune diseases. In contrast to chemically synthesized molecules, biologicals are large and complex due to their natural origin with complicated synthesis and purification processes making biological products much more expensive than synthetic drugs.
To improve access to biological products at lower costs, the United States passed the Biologics Price Competition and Innovation (BPCI) Act for the development of so-called ‘generic’ versions of biologicals known as biosimilars. Owing to the structural complexity and natural origin, it is not possible to produce identical copies of biologicals unlike chemically synthesized drugs, thus biosimilars are ‘highly similar’ to innovator biologicals.
The term ‘biosimilarity’ means that the biological product is highly like the reference product notwithstanding minor differences in clinically inactive components and that there are no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency of the product. Thus, demonstration of biosimilarity is based on an arsenal of techniques including analytical characterization, nonclinical studies, clinical pharmacology (pharmacokinetic/pharmacodynamic) studies, and clinical studies (safety, efficacy, and immunogenicity) using a stepwise approach to biosimilar development. Although the extent and type of clinical studies for biosimilars varies depending upon the residual uncertainty following analytical and nonclinical studies, clinical trials are nonetheless essential in proving safety and efficacy.
In addition, a comparison of immunogenicity between innovator biologicals and biosimilars is critical as biologicals are proteins and can elicit different immune responses depending upon manufacturing and biotechnology processes used to produce them.
The various types of clinical studies for biosimilars are shown in Figure 1 below:
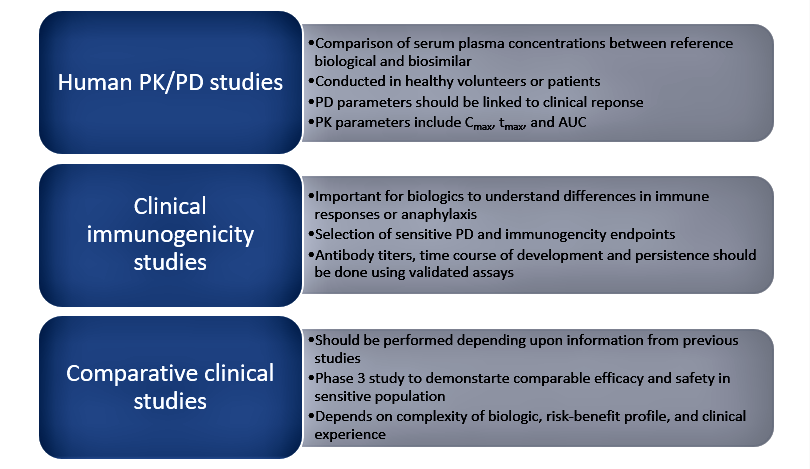
Other clinical studies relevant to biosimilars include switching studies that evaluate the impact of transitioning patients between reference and biosimilar products and evaluate safety, efficacy, and immunogenicity. Extrapolation studies can also be done to demonstrate the use of biosimilars for other clinical indications held by the reference product and bridging studies that are done to demonstrate similarity in specific patient populations.
Study designs considerations for biosimilar Studies
Clinical trials for biosimilars are not meant to establish the clinical effectiveness of the biosimilar product. The aim is to instead to demonstrate similar clinical efficacy between the biosimilar and innovator/reference biological based on the assumption that the innovator product has demonstrated evidence of efficacy and safety in a previous development program. Keeping in mind this overarching goal, the FDA and European Medicines Agency (EMA) have released guidance documents highlighting their recommendations on the study designs that should be used to demonstrate biosimilarity as well as other factors such as sample size, duration of the study, selection of endpoints, and study population that are suitable for biosimilarity studies.
Parallel or crossover designs
Parallel study designs are preferred for biosimilar clinical studies as they involve the administration of the treatments (innovator biological and biosimilar) to two different groups of patients with matched demographic characteristics at the same time in a randomized manner. In contrast, crossover designs involve the administration of both treatments to the same patient at two different times with a washout period between them. Since most biologics have a long half-life, the washout period between the treatments may be extremely long and impractical. Crossover designs are not suitable when the disease is not stable as is the case with inflammatory diseases and associated with flare-ups. Parallel designs require fewer patients to achieve the same statistical power as crossover studies and are more ethical in nature furthermore, statistical analysis is also simpler when comparing two independent patient groups without confounding factors. The FDA and EMA guidelines recommend the use of parallel designs for immunogenicity assessment in treatment-naïve patients as it is the most sensitive design.
Equivalence or noninferiority study designs
The type of study design chosen whether equivalence, superiority, or noninferiority depends on the clinical indication, treatment effect size, availability of clinical data, and regulatory requirements. Since biosimilarity studies are intended to demonstrate the similarity between the reference biological and biosimilar, equivalence designs are preferred by most regulatory bodies.
The null hypothesis for equivalence trials is that the biosimilar is either superior to or inferior to the reference product based on a prespecified equivalence margin. The equivalence margin is defined based on historically and scientifically justified differences in treatment effects. Although symmetric superiority and inferiority margins are most used, asymmetric margins may be used in cases on non-dose related toxicities as they require smaller sample sizes. The most difficult aspect of equivalence study designs is defining the equivalence margin as it should be accepted by regulators, depends on the therapeutic indication and the efficacy endpoint, and determines the sample size. However, regulatory bodies do not specify equivalence margins such that this aspect needs careful consideration.
In addition to equivalence studies, the FDA and EMA also accept the use of non-inferiority study designs provided that their use is appropriately justified. Non-inferiority studies require a smaller sample size than equivalence designs and their use is justified in cases where the reference biological is used close to its maximal level of clinical effect.
The null hypothesis in these studies is that the biosimilar product is inferior to the reference drug based on a pre-specified equivalence margin. However, the conclusions from noninferiority studies should be interpreted with caution as it cannot exclude the increased activity of the biosimilar which may be associated with greater adverse events. Therefore, in most cases, equivalence designs are more rigorous and should be used as a default for biosimilars unless appropriate justification is provided to use an alternate study design.
Although regulatory authorities provide guidance on endpoints, sample sizes, and study designs, they do not provide specific equivalence/noninferiority margins that should be used or even interpretation of results from equivalence and non-inferiority studies.
Thus, it is extremely important for sponsors/investigators to discuss all aspects of biosimilar study designs with regulatory authorities to allow for the approval of biosimilars which will increase access to biologics at a lower cost. Sponsors should use scientific justification from historical data to support all aspects of study design and if necessary, discuss their proposed plan with regulators to ensure smooth progress of their clinical trial.
Read More:
Changes to CDSCO’s Rules for Clinical Trials in India: New Drugs & Clinical Trial Rules 2019
References
- Clinical trial development for biosimilars – ScienceDirect
- Clinical trial design in biosimilar drug development – PubMed (nih.gov)
- Key design considerations on comparative clinical efficacy studies for biosimilars: adalimumab as an example – PubMed (nih.gov)
- Scientific Considerations in Demonstrating Biosimilarity to a Reference Product Guidance for Industry (fda.gov)
- Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues (europa.eu)




