Although the United States Food and Drug Administration (FDA) has always supported and advocated the idea of decentralized trials, the real adoption and application of decentralized aspects in trials has exponentially increased in the recent years due to travel restrictions, social distancing measures, and site closures caused by the Covid-19 pandemic.
The pandemic caused several aspects of trials such as patient recruitment, monitoring, follow-up visits, and investigational product dispensing to be conducted remotely. Furthermore, advances in digital technologies and improved internet access globally have also led to the natural growth in the use of decentralized aspects. Along with patient convenience and comfort, remote conduct of trials has a plethora of benefits which include:
- Ability to recruit a greater number of participants and a more diverse population.
- Enables the study of rare diseases by allowing for a suitable number of participants for these conditions that have fewer affected patients.
- Allows elderly patients and those who are immobile to participate in trials.
- Decreased costs associated with maintenance of sites and staff expenses.
- Ability to collect data in real-time using patient feedback that allows for better control of safety and adverse events.
To enable sponsors/investigators to understand FDA’s stance on decentralized trials, the FDA released a guidance document in May 2023 entitled, “Decentralized Clinical Trials for Drugs, Biological Products, and Devices: Guidance for Industry, Investigators, and Other Stakeholders”. In this guidance, the FDA defines fully decentralized clinical trials (DCTs) as those in which all activities take place at locations (patient’s homes, local healthcare facilities) other than traditional trial sites and hybrid DCTs are those in which some trial activities are conducted at trial sites, and some are conducted remotely.
Although, the requirements for DCTs remain the same as for traditional trials, this recent document highlights some important points pertaining to DCTs namely, the type of drug administration and safety profiles for which decentralized designs can be used, technologies that facilitate remote patient-physician interactions such as telehealth platforms, and digital health technologies that allow data to be obtained remotely and continuously from patients allowing for improvement in patient recruitment, enrolment, engagement, and retention. Overall, better patient engagement allows for improved data quality and better outcome from clinical trials especially for rare diseases and those in which patient populations have limited mobility.
Despite the several advantages associated with DCTs their uptake is limited by challenges such as coordination of activities at multiple sites, training of heath care providers and trial coordinators, and patient hesitation and comfort with remote visits. Thus, this updated guidance attempts to address these issues by considering various aspects of DCTs and shedding light on regulatory requirements such that sponsors can implement these when planning and executing decentralized elements in their clinical trials.
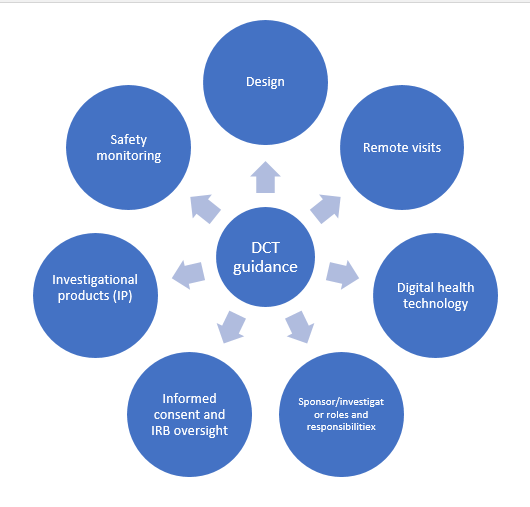
The new guidance covers various aspects of DCTs as shown in the Figure below:
Design:
Since some activities in a decentralized trial may be conducted at locations other than a central site, it is reasonable to expect variability in assessments which are often performed by patients or local healthcare providers (HCPs), which can affect the precison and variability of data. Therefore, it is important to adjust effect sizes and margins especially for non-inferiority trials and require consultation with FDA review divisions prior to planning.
Remote Visits:
Remote visits improve patient convenience and accessibility and can be performed in several ways such as telehealth visits when in-person interaction is not critical, visits by local HCPs who are not part of the trial personnel and they should perform routine activities not requiring in-depth knowledge of the protocol or IP, or remote visits by qualified trial personnel when expertise and detailed knowledge of the protocol or IP are necessary. It is essential to confirm the trial participants identity at each remote visit and ensure completion of case report forms (CRFs). Adverse event evaluation and management should also be specified in the protocol to ensure that safety is maintained.
Digital health technology (DHT):
DHTs allow for transmission and collection of data from patients remotely via wearable sensors or other remote monitoring devices. ‘Digital Health Technologies for Remote Data Acquisition in Clinical Investigations’ draft guidance provides details on measuring characteristics using DHTs including verification, validation, usability testing, training, and management of risks related to DHT use. It is the responsibility of the sponsor to ensure that DHTs are suitable and available for use by all trial participants to allow access to lower socioeconomic groups in trials.
Sponsor/investigator roles and responsibilities:
In addition to the responsibilities of sponsors as in traditional trials, sponsors of DCTs have other responsibilities which are specific to decentralized activities as well which include proper coordination of contracted services, expanded recruitment of diverse populations especially in areas that lack traditional trial sites thereby increasing diversity and inclusion in trial participant representation, employment and use of local HCPs, formulation of a data management plan (DMP) that involves a data flow representation to the sponsor, list of vendors, and methods for remote data acquisition.
Sponsors are required to describe operational aspects of the trial in the protocol such as trial visits, IP delivery, safety monitoring and adverse event management, transmission of reports, and a comprehensive trial monitoring plan in accordance with local laws and regulations. Like sponsors, investigators should ensure management of telehealth, local HCPs, and DHTs in trial conduct. Investigators are responsible for adequate trial participant enrolment and retention, subinvestigator inclusion, quality control measures to reduce variability in local HCP practices and activities, maintenance of a task log of HCPs, comprehensive listing of all local laboratory facilities in the appropriate FDA forms and investigational plans, and obtaining health reports from local health facilities and primary providers to keep records of health emergencies or changes in concomitant medications, etc.
Informed consent & institutional review board (IRB) oversight:
Informed consent can be obtained remotely in DCTs through electronic forms provided all regulatory requirements regarding informed consent are met. It is necessary to inform the participants on appropriate contact persons for queries or safety issues during the trial as well as keep them informed on personnel who will have accessibility to their personal health information. The FDA suggests employing a central IRB to review the protocol, informed consent materials, and any other information related to clinical trials conducted in a decentralized manner.
Investigational products:
The nature, administration procedure, safety profile, trial type, and risks associated with a drug or medical device determine its administration by local HCPs or trial personnel and complexity of care that may be required with its use. Drugs that are simple to administer and do not require close supervision following administration can be administered remotely by local HCPs whereas those that have a complex administration routine and high-risk profile often require on-site administration. Stability profile and storage conditions for the drug dictate whether it can be shipped to homes or local clinics or need to be kept at trial sites.
The type of medical device, its intended use, instructions for use, and risk profile determines whether it is suitable for self-use or requires investigator oversight. In case where the IP needs to be directly shipped to trial participants at their homes or local healthcare centres, the protocol should state appropriate packaging requirements to maintain the integrity and stability of the product, tracking information, return and disposal protocol for unused IP, and central shipping and distribution services information.
Safety monitoring plan:
A suitable safety monitoring plan is necessary in DCTs to capture information on adverse events and specify their management, and should include information for participants on where to seek medical assistance in case of emergencies, report adverse events, and arrange unscheduled visits. It should also include the type of information to be collected by DHTs and the use of the information as well as contact information in case of abnormal signals or alerts from DHT devices. Discontinuation of remote IP administration should be done in case of safety risks and the IRB and FDA, and participants should be informed of the same.
This following aspects should be considered and understood by sponsors/investigators planning to use remote technology and visits in their clinical trial plan to ensure meaningful information is obtained from the trial and that the rights and welfare of the participants is protected.
Read More: Biosimilar Clinical Trials and US FDA Guidance
References
1) Decentralized Clinical Trials for Drugs, Biological Products, and Devices | FDA
2) Digital Health Technologies for Remote Data Acquisition in Clinical Investigations | FDA