The rising prevalence of ophthalmology disorders such as dry eye disease (DED) and vision-related issues is largely related to the increasing use of mobile phones, tablet screens, and laptops. Since the adoption of technology is ever increasing from children to the elderly, it becomes necessary to develop suitable treatments for the management of ophthalmic disorders. Most of the common vision-related issues, cataracts, refractive errors (myopia, hyperopia, astigmatism, presbyopia), amblyopia (lazy eye), and blindness are managed or treated surgical procedures whereas those such DED, macular degeneration, neurotropic keratitis, and diabetic macular edema involve the use of targeted drugs. The ophthalmology treatment segment is expected to show a rapid growth driven by lifestyle changes, reimbursement policies, increased longevity, and increased access to ophthalmic procedures. Despite this these disorders place a significant burden on patients by affecting their daily life and their inability to participate in several tasks. Healthcare costs associated with diagnostic procedures, rehabilitation requirements, and medical treatment is also substantial requiring the development of advanced procedures for early detection and treatment.
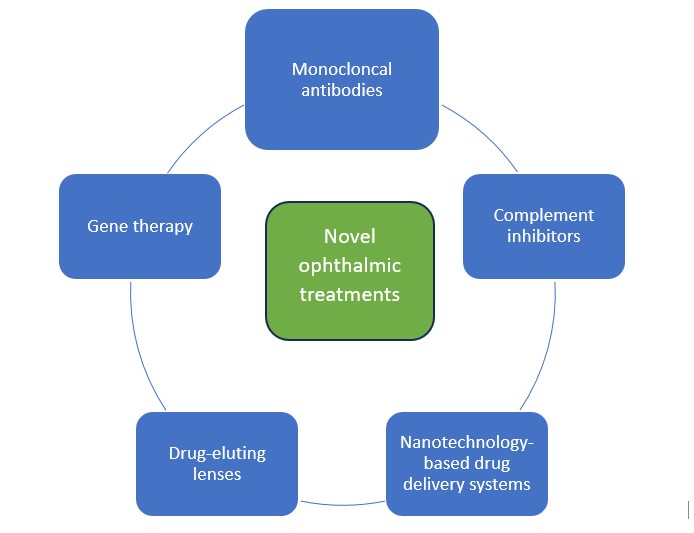
Traditional ophthalmic treatments although effective may cause side effects that can range from mild to severe, can have limited efficacy especially with progression of the disorder or individual variability, develop resistance to treatment, and may not have the desired outcome. The objective of novel, targeted ophthalmic treatments is to provide better outcomes in cases of disease progression, reduce side effects, and improve patients’ quality of life. Other than invasive surgical procedures, novel drug treatments are being developed with several drugs in the pipeline for common ophthalmic disorders such as DED, macular degeneration, diabetic retinopathy, neurotrophic keratitis, and meibomian gland dysfunction as shown in the figure below.
Some of the recently approved drugs for ophthalmology disorders by the United States Food and Drug Administration (FDA) from 2020-2023 include:
- Faracimab, a bispecific monoclonal antibody for wet age-related macular degeneration (AMD)
- Tenentafusp, a bispecific protein for metastatic uveal melanoma.
- Ketotofin, a drug-eluting contact lens for allergic conjunctivitis.
- Brolucizumab, a humanized monoclonal antibody for diabetic macular edema.
- Avacincapted pegol intravitreol solution, a C5 complement inhibitor for geographic atrophy secondary to AMD.
- Syfovre, a complement inhibitor for dry AMD.
- Flibercept, a vascular endothelial growth factor (VEGF) and placental growth factor (PGF) antagonist for neovascular wet AMD.
- Omidenepag isopropyl, selective E-prostanoid subtype 2 (EP2) agonists for glaucoma and ocular hypertension.
- Perfluorhexyloctane, a semifuorinated alkane used to treat signs and symptoms of DED.
- ACUVUE® Theravision drug-eluting contact lens with ketotifen, an antihistamine drug for the prevention of ocular itch due to allergic conjunctivitis.
Monoclonal antibodies (mAbs):
These target specific proteins or cells and thus reduce side effects associated with common medications and act again various targets such as tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF) receptor, epithelial growth factor receptors, platelet-derived and fibroblast growth factor receptors. The most common mAbs are those that target VEGF and prevent the abnormal growth of blood vessels in the retina that can obstruct vision. Some examples include ranibizumab, bevacizumab, and aflibercept used in the treatment of AMD. This class of drugs are also used for the management of other ophthalmic disorders such as diabetic retinopathy, macular edema, uveitis, allergic conjunctivitis, and retinal vascular disorders.
Complement inhibitors
The complement system plays a vital role in inflammation and immune response and under normal circumstances helps maintain homeostasis in the ocular environment. However, when overstimulated it can lead to ocular inflammatory diseases such as AMD. Thus, complement inhibitors are a class of drugs that target the modulation of complement proteins, C3, C5, factor B, factor D, and properidin and reduce inflammation and tissue damage in the eye.
Nanotechonoly
Based drug delivery systems: Nanoparticulate drug delivery systems offer several advantages by providing targeted and localized delivery to the eye thus avoiding side effects, increased efficacy, and improved bioavailability. Various types of nanotechnology-based delivery systems can be tuned to provide controlled and sustained drug delivery and be designed to carry a variety of drug molecules such as hydrophobic drugs and include polymer-based nanoparticles, nanomicelles, liposomes, quantum dots, nanoemulsions, dendrimers, and inorganic nanoparticles.
Drug-eluting lenses
These contain drugs loaded into contact lenses and allow for release of the drug directly onto the eye surface. They serve as an attractive alternative to eye drops that need to be instilled frequently into the eye providing for improved patient adherence and increased convenience as they allow for a sustained release of the drug. They are used particularly for DED, glaucoma, and allergic conjunctivitis.
Gene therapy
This treatment involves the delivery of functional genes to correct defective or mutated genes and are useful for the treatment of inherited retinal diseases such as Leber congenital amaurosis and retinitis pigmentosa. An FDA-approved retinal gene therapy product is Luxturna™ for inherited retinal disorders in patients with mutations in the RPE65 gene.
The table below enlists some drugs that are under clinical trials in USA (United States) for the treatment of some of the most common ocular disorders-namely, DED, AMD, allergic conjunctivitis, and diabetic macular edema.
| Drug/treatment | Type of treatment/drug | Indication | Phase of clinical development |
| Reproxalap | Small molecule RASP inhibitor | DED | NDA |
| Reproxalap | Small molecule RASP inhibitor | Allergic conjunctivitis | 3 |
| TP-03 | Antiparasitic | Demodex blepharitis | NDA |
| AZR-MD-001 | Selenium sulfide | Meibomian gland dysfunction | 2b |
| RGN-259 | Thymosin beta-4 activator | Neutrophic keratitis | 3 |
| AR-15512 | Transient receptor potential melastatin 8 agonist | DED | 3 |
| SURF-200 | Steroid, betamethasone | Acute dry eye | 2 |
| GLK-301 | Pilocarpine | DED | 2 |
| RGX-314 | VEGF inhibitor | Wet AMD | 3 |
| APX 3330 | Small molecule redox factor 1 and APE | Diabetic retinopathy, diabetic macular edema | 2 |
| Ixoberogene soroparvovec | Gene therapy | Wet AMD | 2 |
| OPT-302 | VEGF inhibitor | Wet AMD | Fast track |
| PAN-90806 | Anti-VEGF | Wet AMD | 2 |
Read More
Successful Clinical Trial With ProRelix Research: Phase 1, 2, 3 & 4 Clinical Trial Services at glance
References
- Complement Inhibitors in Age-Related Macular Degeneration: A Potential Therapeutic Option – PMC (nih.gov)
- Johnson & Johnson Vision Care Receives FDA Approval for ACUVUE® Theravision™ with Ketotifen – World’s First and Only Drug-Eluting Contact Lens (jjvision.com)
- The Challenges and Promise of Complement Therapeutics for Ocular Diseases – PubMed (nih.gov)
- Gene therapy in ophthalmology – PMC (nih.gov)
- Frontiers | Nanoparticles in ocular applications and their potential toxicity (frontiersin.org)
- Therapeutic monoclonal antibodies in ophthalmology – PubMed (nih.gov)