COVID 19 clinical trials and emergency marketing approval process in India – Overview
The COVID-19 pandemic has transformed the worldwide regulator performing on approval of medicine as a fast-track process and involved in mutual accord in bringing a drug of importance for the civic. This is a major challenge as the safety and effectiveness of novel therapies are not fully established but ways to bring about a difference to the COVID-19 pandemic response is the need of the hour. Regulators have been go-getting hard in order to bring flexibility in the regulations so as to quicken various approval and adaptive licensing mechanisms.

Various institutions have been working together in an alliance to fight this pandemic. The WHO has always promoted such cooperative approaches as these enhance the efficiency of regulatory authorities and also avoids copying. We have focused on details of clinical trials and diverse classes of drugs that are suitable to treat COVID 19 infection.
Coronavirus disease (COVID-19) is a transmittable disease caused by Coronavirus. Most people are infected with the COVID-19 virus would experience mild to moderate respiratory illness and recover without requiring special treatment. Older people with medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop severe illnesses.
The best way to prevent transmission is to be well informed about the spread of the COVID- 19 viruses. The COVID-19 virus spreads mainly through saliva or from the nose when an infected person coughs or sneezes.
The “Emergency use authorization” term is not used in India as per 2019 vaccine approval. A window of “accelerated approval” is used in “special situations”. The Covid-19 pandemic is one such special situation.
The New Drugs & Clinical Trial Rules 2019 in India say, “Accelerated approval may also be granted to a new drug if it is intended for the treatment of a serious, or life-threatening condition, or disease of unusual relevance to the country, and addresses unmet medical requirements. “There is a special provision for accelerated approval of vaccine if the phase-II of clinical trial result show “remarkable efficacy”.
According to New Drugs & Clinical Trial Rules 2019 in India: “If the remarkable efficacy is observed with a defined dose in the phase-II clinical trials of the investigational new drug for the un-met medical emergency of serious and life-threatening disease in the country, it may be considered for grant of marketing approval by the central licensing authority based on phase-II clinical trial data.”
The Central Drugs Standard Control Organization (CDSCO) has the authority of granting approval for a vaccine or any other drug rests, headed by the Drug Controller General of India (DCGI), a works under the Union health and family welfare ministry.
COVAXIN Approval:
The DCGI stated that COVAXIN was “developed on Vero cell platform, which has a well- established track record of safety and efficacy in the country and globally”.
The DCGI also stated that COVAXIN developer, Bharat Biotech has shared its “safety and immunogenicity data” from its trials on animals including “challenge studies on non-human primates (Rhesus macaques) and hamsters” with the CSDSCO.
Human clinical trials – The DCGI stated for phase-I and phase-II “demonstrated that the vaccine is safe and provides a robust immune response”. Mentioning phase-III data from around 22,500 participants, the drug regulator said, “the vaccine has been found to be safe as per the data available to date.”
The DCGI stated that “before recommending grant of permission for restricted use of this COVID-19 vaccine in an emergency situation”, the Subject Expert Committee (SEC) of the CSDSCO reviewed the data on the safety and immunogenicity of the COVAXIN.
How India approves vaccines?
The science of vaccine development is universal, the regulatory process for its use and authorization varies in each country. According to New Drugs and Clinical Trial Rules – 2019 of India steps need to be followed for approval of vaccine or drug.
Under usual circumstances, the approval for a Vaccine or New Drug follows the following steps:
- Identification and development of suitable vaccine strain that is actually safe and immunogenic.
- Full characterization of the vaccine strain by in-vitro
- Pre-clinical studies in small animals like mice, rabbits, guinea pigs, etc to work out safety and dose
- Preclinical studies in large animals to determine safety, protective efficiency, and potential dose and
- Phase 1 human clinical trials on less than 100 individuals to determine the safety of the
- Phase 2 human clinical trials on usually less than 1,000 individuals to work out the immunogenicity or immune
- Phase 3 human clinical trials to work out the efficacy. The numbers range in several thousand. After the successful completion of phase III studies, regulatory approval is accorded.
“Phase 4 human clinical trials refer to post-marketing surveillance studies, whose data are analyzed for long-term decision-making”. “A key aspect of granting approval to a vaccine or a drug in India is the requirement of clinical trials conducted within the country from phase 1 to Phase 3”.
How “2DG” COVID-19 Tablets Released in Market?
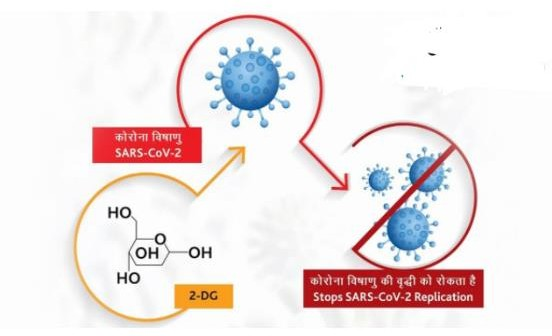
The 2-deoxy-D-glucose drug, the first such medicine in India meant to treat COVID-19 infections. The anti-COVID therapeutic application of the drug has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a leading laboratory of the DRDO, in collaboration with Dr Reddy’s Laboratories (DRL) in Hyderabad.
The Drugs Controller General of India (DGCI) has approved the oral drug for emergency use as an adjunct therapy in moderate to severe coronavirus patients. The approval of the drug has come at a time when India is grappling with a record-breaking wave of the coronavirus pandemic that has pushed the country’s healthcare infrastructure to its limit.
DRDO Chairman Dr G Satheesh Reddy said that “DRDO and Dr Reddy’s lab had gone through the complete trials and conducted trials across 30 hospitals and on a large number of patients.”
The drug comes in the precipitate form in a sachet, which can be taken orally by dissolving it in water. It accumulates in the virus-infected cells and prevents virus growth by stopping viral synthesis and energy production. Its discriminatory accumulation in virally infected cells makes this drug unique.
Clinical trial results have shown that this drug helps in the faster recovery of hospitalized patients and reduces supplemental oxygen dependence. A higher proportion of patients treated with 2-DG showed RT-PCR negative conversion in COVID patients.
In April 2020, during the 1st wave of the pandemic, INMAS-DRDO scientists conducted laboratory experiments with the help of Center for Cellular and Molecular Biology (CCMB), Hyderabad, and found that this molecule works effectively against the SARS-CoV-2 virus and inhibits viral growth.
May 2020 – Based on the results, the CDSCO) allowed Phase-II clinical trial of 2- DG in Covid-19 patients. The DRDO, along with its industry partner DRL, Hyderabad, started the clinical trials to test the safety and efficacy of the drug in Covid-19 patients.
May-October 2020 – Phase-II trials (including dose-ranging), the drug was found to be safe in Covid patients and showed significant improvement in their recovery, the ministry statement said adding, “Phase-II was conducted in six hospitals and Phase IIb (dose-ranging) clinical trial was conducted at 11 hospitals all over the country. Inefficiency trends, the patients treated with 2-DG showed faster symptomatic cure than “Standard of Care (SoC)” on various endpoints. A significantly favorable trend (2.5 days difference) was seen in terms of the median time to achieving normalization of specific vital signs parameters when compared to SoC.”
November 2020 – Phase-3 trials were approved – from December to April. DCGI further permitted the Phase-III clinical trials
May 1 – The drug was approved for emergency use. Dr Reddy’s Labs is engaged in its production. Based on successful results, in November 2020.
December 2020 to March 2021 – The Phase-III clinical trial was conducted on 220 patients between at 27 Covid hospitals in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka and Tamil Nadu. The detailed data of phase-III clinical trial was presented to DCGI. In 2-DG arm, a significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by Day-3 in comparison to SoC, indicating an early relief from Oxygen therapy/dependence. On May 01, 2021, DCGI granted permission for Emergency Use of this drug as an adjunct therapy in moderate to severe Covid-19 patients.
References:
- https://www.ijponline.com/article.aspissn=02537613;year=2020;volume=52;issue=6;spage=457;epage=466;aulast=Mahalmani#ref20
- https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/covaxin-approval-row-how-india-approves-vaccines-explained-1756049-2021-01-05
- https://www.news18.com/news/india/as-india-waits-for-covid-19-shot-a-look-into-accelerated-approval-for-vaccines-and-how-its-cleared-3155603.html
- https://www.hindustantimes.com/india-news/drdos-2dg-medicine-to-treat-covid-19- availability-dosage-price-10162html





