Pharmacovigilance is defined as the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects and other drug-related problems. It is an important component of the post-marketing surveillance program of medicinal products and medical devices as it helps monitor adverse effects (AE), adverse drug reactions (ADR), and any other safety concerns that were not captured during clinical trials. Safety data from pharmacovigilance activities is critical as it gives information about the benefits/side effects of the drug in a diverse patient population and helps detect any new and previously unreported risks. Although several pharmacovigilance systems are in place and most companies employ pharmacovigilance activities, it is often possible to miss some important safety information and underreporting is common. The FDA pharmacovigilance systems voluntary reporting systems such as MedWatch for consumers and healthcare professionals and VigiBase used by the World Health Organization (WHO), reporting by sponsors, and mandatory reporting by manufacturers involves data collection to the FDA Adverse Event Reporting Systems (FAERS) database which then reaches the FDA to make decisions on new warning labels or withdrawal/limited use of the product in the market.
Although pharmaceutical manufacturers, sponsors, and patient advocacy groups have realized the importance of good pharmacovigilance practices, pharmacovigilance activities and reporting continue to remain plagued by challenges. Underreporting, misreporting, missing and incomplete information, and inconsistences in safety data are considered common as they are entered into systems both voluntarily and in a non-standardized manner. Analysis of trends and patterns from a large volume of data is also difficult if not handled by advanced analytical techniques to draw meaningful conclusions. Thus, digital health technologies (DHT) are an important tool to improve pharmacovigilance by enabling collection and analysis of adverse events and safety data.
Digital health technologies (DHT) use computing platforms, connectivity, software, and sensors for healthcare and related uses. They include mobile medical apps (mHealth), artificial intelligence (AI), and machine learning (ML), wearable devices, telehealth and telemedicine, and personalized medicine.
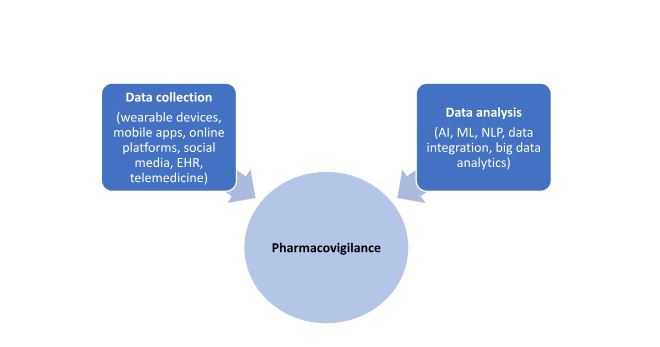
There are two major ways in which DHTs are applicable in pharmacovigilance as shown in the Figure below:
Figure. Impact of DHT on Pharmacovigilance
Data collection
Wearable devices
DHTs such wearable devices including mobile phones, smart watches, fitness trackers, and mobile apps involve real-time collection and monitoring of data allowing adverse events to be detected, identified, and categorized as soon as they occur. This allows regulatory authorities and manufacturers to take immediate action and mitigate any potential risks.
Online platforms and social media
Online communities and social media websites serve as an important repository of safety concerns and information that are entered in by patients, advocacy groups, and caretakers. This information is important as it is entered directly in by patients and can help identify trends in safety data for certain drug classes and medical devices.
Electronic health records (EHRs)
EHRs are an important source of data for detection of adverse drug reactions and allow for cross-functional sharing of information with network of healthcare professionals enabling tracking and remediation of safety information. EHRs contain more detailed information such as timing of medication administration, symptom development, and detailed clinical history which may be of interest in understanding adverse events in a comprehensive manner.
Telemedicine
Telehealth platforms which involve remote visits and monitoring of patients without the need for in-person consultations are important for identification and communication of safety concerns by patients in a timely manner. This method allows physicians to understand adverse events and take appropriate adjustments such as stoppage of medication or dosage adjustments and can be integrated with EHRs.
Data analysis
Artificial intelligence (AI) and machine learning (ML)
These technologies serve to go through large amounts of data generated from wearable devices and process it to identify trends and patterns in safety data. AI allows for processing of pharmacovigilance reports and signal detection to extract information about potential adverse drug events. Data mining and prediction using AI and ML is also done on data obtained from social media platforms. ML techniques can be used to predict the probability of developing an adverse event based on patient history and characteristics and helps in the development of predictive models.
Data integration
Technologies such as AI and cloud computing are important in the field of pharmacovigilance which involves data extraction and analysis from a wide variety of sources into a single dataset to identify trends in safety information. These technologies are also important in ensuring data security and privacy.
Natural Language Processing (NLP)
This system helps extract structured safety and adverse event information from text-based documents such as EHRs, narrative reviews, and social media platforms.
Big data analytics
This is important for processing vast amounts of data and uses algorithms to extract meaningful insights regarding safety concerns linked to use of drugs or medical devices. It involves integration of diverse data form different sources to ascertain the causes of adverse events and is particularly useful when the data comes from unstructured sources such as (real-world evidence) RWE studies.
Therefore, application of DHT is a vital component of pharmacovigilance practices allowing more data to be handled in an accurate and timely manner which facilitating results in safety information to be identified rapidly and swift action by regulatory authorities to safeguard patient health.
Read More: Pharmacovigilance (PV) Services at a Glance
References
- What is Digital Health? | FDA
- Pharmacovigilance Analytics – Pharmacovigilance Analytics
- Natural Language Processing for EHR-Based Pharmacovigilance: A Structured Review – PubMed (nih.gov)
- Transforming Drug Safety: The Future of Pharmacovigilance and Technology (pharmaceuticalcommerce.com)