Discover how eCOA in clinical trials transforms data collection and uncover the game-changing benefits of electronic data capture in clinical research.
Introduction: What is eCOA in Clinical Trials?
eCOA in clinical trials are a type of clinical research in the field of healthcare and medicine. eCOA refers to the use of electronic methods and technologies to collect patient-reported outcomes and other clinical data in a clinical trial setting. These electronic methods can include the use of smartphones, tablets, wearable devices, web-based platforms, or other digital tools to record and transmit information about a patient’s symptoms, experiences, and overall health status.
The primary goal of eCOA clinical trials is to improve the accuracy and efficiency of data collection during clinical research. By using electronic tools, researchers can reduce the potential for errors associated with manual data entry and streamline the data collection process, making it more convenient for both patients and investigators. eCOA methods are often used to assess patient-reported outcomes such as quality of life, symptom severity, and treatment-related side effects.
Significance of eCOA in Clinical Trials
eCOA, or Electronic Clinical Outcome Assessment, has emerged as a pivotal tool in modern clinical trials. eCOA in Clinical Trials offers numerous advantages, enhancing data accuracy and patient engagement, making it a fundamental aspect of contemporary research methodologies. By enabling patients to input their health-related information directly into electronic devices, eCOA minimizes transcription errors and streamlines data collection, ultimately improving the quality of trial outcomes. Furthermore, eCOA’s ability to facilitate remote data capture has become especially vital in the context of global health crises, ensuring trial continuity and participant safety. As we navigate the evolving landscape of clinical research, eCOA Clinical Trials remains at the forefront, revolutionizing the way we gather and utilize critical data.
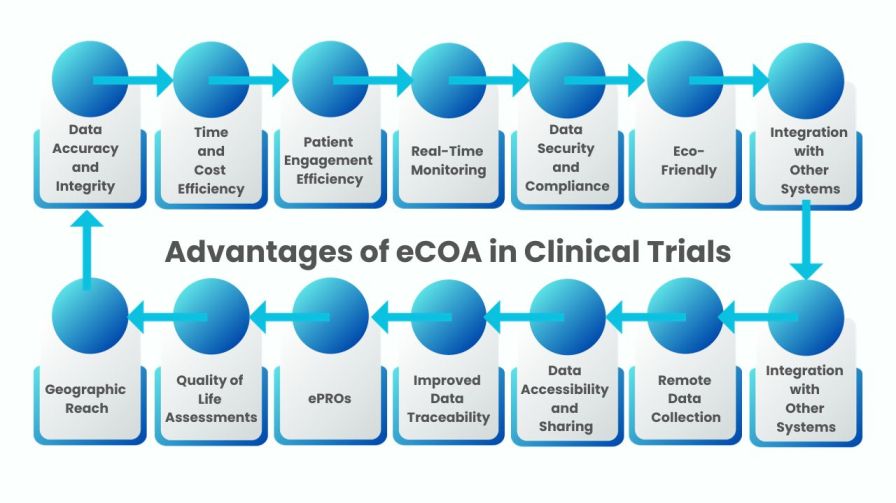
The Advantages of eCOA in Clinical Trials over Traditional Paper-Based Data Collection Methods
The advantages of Electronic Clinical Outcome Assessment (eCOA) over traditional paper-based data collection methods presented in bullet points:
Figure: The Advantages of eCOA in Clinical Trials over Traditional Paper-Based Data Collection Methods
Data Accuracy and Integrity
- Reduced risk of transcription errors and data entry mistakes.
- Real-time data validation and automated skip patterns enhance data quality.
Time and Cost Efficiency
- Eliminates the need for printing, shipping, and storing paper forms.
- Faster data collection, review, and analysis, leading to shorter study timelines.
Patient Engagement
- User-friendly interfaces improve patient compliance and satisfaction.
- The ability to collect data remotely encourages broader patient participation.
Real-Time Monitoring
- Researchers can access data instantly, enabling prompt response to issues.
- Early identification of adverse events or protocol deviations.
Data Security and Compliance
- Enhanced data encryption and audit trails protect sensitive patient information.
- Adherence to regulatory standards (e.g., 21 CFR Part 11) is facilitated.
Eco-Friendly
- Reduces paper waste, contributing to a greener and more sustainable approach.
- Aligns with environmental responsibility goals.
Integration with Other Systems
- Easily integrates with Electronic Data Capture (EDC) and other clinical trial systems.
- Streamlines data flow and enhances the overall efficiency of clinical trials.
Remote Data Collection
- Allows for remote patient monitoring and data collection, reducing the need for physical site visits.
- Particularly valuable during global health crises (e.g., pandemics) to maintain data collection continuity.
Data Accessibility and Sharing
- Facilitates secure data sharing among stakeholders, including sponsors, CROs, and regulatory authorities.
- Enhances transparency in the research process.
Reduced Administrative Burden
- Minimizes the need for manual data handling, reducing administrative workload.
- Automates data collection and management tasks.
Customization and Adaptability
- Easily configurable to specific study requirements.
- Can accommodate a wide range of study types and data formats.
Improved Data Traceability
- Easy tracking of data changes and user interactions, aiding in the audit trail.
- Enhances the ability to reconstruct data history if needed.
Enhanced Patient-Reported Outcomes (ePROs)
- Supports the collection of patient-reported outcomes more efficiently and accurately.
- Provides a patient-centric approach to data collection.
Quality of Life Assessments
- Allows for real-time assessment of quality of life measures, particularly important in long-term studies or chronic disease research.
Geographic Reach
- Overcomes geographical barriers, enabling participation of patients from diverse locations.
- Facilitates multi-site and global clinical trials.
These advantages demonstrate how eCOA methods offer a significant improvement over traditional paper-based data collection in clinical research, leading to more efficient, reliable, and patient-centric outcomes.
How eCOA in Clinical Trials Enhances Patient Engagement and Retention?
Electronic Clinical Outcome Assessments (eCOA) have revolutionized the way clinical trials are conducted, offering a range of benefits, including the enhancement of patient engagement and retention. Patient engagement and retention are critical factors in the success of clinical trials, as they directly impact the quality and reliability of the data collected. Here’s how eCOA achieves this:
Convenience and Accessibility
eCOA platforms provide patients with the flexibility to complete assessments from the comfort of their own homes, reducing the need for in-person visits. This convenience makes it easier for patients to participate in the trial, especially those who may have mobility issues or live far from trial sites. As a result, patients are more likely to stay engaged throughout the trial’s duration.
Real-time Communication
eCOA systems enable real-time communication between patients and researchers. This means that patients can quickly report any issues or concerns, fostering a sense of being closely monitored and cared for. This transparency can improve patient satisfaction and trust in the clinical trial process.
User-Friendly Interfaces
eCOA platforms often come with user-friendly interfaces that are easy for patients to navigate. This reduces the burden on patients, making it less likely for them to become overwhelmed or frustrated with the assessment process. A positive user experience encourages patients to stay engaged in the trial.
Automated Reminders
eCOA systems can send automated reminders to patients, prompting them to complete assessments on time. This minimizes the risk of missed assessments and improves data reliability. Patients are more likely to remain engaged when they receive gentle reminders about their responsibilities in the trial.
Customization
eCOA platforms allow for the customization of assessments to better match a patient’s condition and individual needs. This personalized approach can make patients feel more valued, enhancing their commitment to the trial.
Data Visualization
Patients can often access their own assessment data through eCOA platforms, allowing them to track their progress. This visibility into their health data empowers patients and keeps them engaged in the study.
Reduced Burden
Paper-based assessments can be burdensome for patients, leading to fatigue and dropout. eCOA streamlines data collection and reduces the need for excessive paperwork. Patients are more likely to remain engaged when the process is simplified.
Safety and Compliance Checks
eCOA platforms can include built-in safety checks and validation rules, which ensure that patients provide accurate and complete data. This enhances data quality and compliance while giving patients a sense that their safety is a priority.
Data Security and Privacy in eCOA Clinical Trials
eCOA systems are designed to maintain the privacy and security of patient data. This assurance can make patients more comfortable with sharing their information, knowing that it will be handled with the utmost care.
Ensuring data security and privacy in electronic Clinical Outcome Assessments (eCOA) clinical trials is critical to safeguard patient information, maintain data integrity, and meet regulatory requirements. To achieve this, adherence to relevant data protection regulations, such as GDPR in Europe and HIPAA in the United States, is paramount. Robust encryption measures for data in transit and at rest should be in place to protect data from unauthorized access.
Strong user authentication, role-based access control, and audit trails help manage and monitor data access effectively. Data minimization practices, secure data storage, and encrypted data transmission are essential for protecting patient information. Furthermore, eCOA device security, oversight of third-party vendors, and informed consent processes are crucial to maintaining data security and patient privacy.
Well-defined data retention and disposal policies, along with a comprehensive data breach response plan, further enhance data protection. Regular training, awareness initiatives, and continuous monitoring ensure that data security remains a top priority throughout the eCOA clinical trial process.
Data Integrity and Compliance in eCOA Clinical Trials
eCOA in clinical trials plays a critical role in ensuring data integrity and compliance in clinical trials. In an era where clinical research is becoming increasingly complex and data-driven, eCOA solutions have emerged as a powerful tool to enhance the quality of data collection, reduce errors, and streamline the regulatory compliance process.
Data Accuracy and Consistency
One of the primary benefits of eCOA is its ability to ensure the accuracy and consistency of data collected during clinical trials. By replacing paper-based assessments with electronic data capture methods, eCOA systems eliminate the risk of transcription errors, missing data, and illegible handwriting that can plague traditional data collection processes. This not only improves the quality of data but also minimizes the need for data cleaning, reducing the time and resources required for this tedious task.
Real-time Data Monitoring
eCOA systems enable real-time data monitoring, allowing sponsors and investigators to review and assess data as it is collected. This capability enhances the ability to detect discrepancies or irregularities promptly, facilitating timely corrective actions. In case of any issues or non-compliance with the trial protocol, immediate intervention is possible, preventing the accumulation of inaccuracies that could compromise the trial’s integrity.
Protocol Adherence
Clinical trial protocols are carefully designed to ensure patient safety, study integrity, and compliance with regulatory requirements. eCOA solutions can include built-in checks and validations to ensure that data collected aligns with the defined protocol. For instance, eCOA systems can flag entries that fall outside predefined ranges, require mandatory fields to be completed, or provide automated skip patterns based on participant responses. This promotes protocol adherence and minimizes deviations that could jeopardize data integrity.
Audit Trails and Data Security
Data integrity and compliance are closely related to data security. eCOA systems provide robust security measures to protect sensitive patient information and research data. Furthermore, these systems often maintain detailed audit trails that record every interaction with the data, including who accessed it, when, and what changes were made. This audit trail is invaluable for regulatory compliance and data integrity, as it allows complete traceability and accountability for all data-related actions.
Regulatory Compliance
eCOA solutions are designed with the latest regulatory guidelines and standards in mind, ensuring that clinical trials are conducted in accordance with Good Clinical Practice (GCP) and other relevant regulations. Data captured through eCOA is more likely to meet regulatory requirements due to its reduced risk of errors and the ability to implement necessary compliance checks. This minimizes the risk of non-compliance, which can result in regulatory sanctions or invalidated trial results.
Patient Engagement and Compliance in eCOA Clinical Trials
The user-friendly interfaces and remote data collection options of eCOA systems make it easier for participants to submit their data accurately and consistently. Patient compliance is crucial in maintaining data integrity, and eCOA can help by simplifying the data collection process for study participants.
The use of electronic methods for data collection in these trials, such as smartphones and web-based applications, not only simplifies the process for patients but also improves data accuracy through validation checks and real-time monitoring. eCOA systems can be designed with a patient-centric approach, offering flexible assessment times and automated reminders, reducing the burden on participants.
Remote monitoring and data collection enable decentralized trials and geographic diversity in recruitment. Privacy and security, compliance with data protection regulations, education, and training are crucial factors to ensure patient trust and effective use of eCOA. Additionally, the feedback loop facilitated by eCOA systems allows patients to report adverse events and concerns directly, contributing to quicker responses and improved patient safety. In eCOA clinical trials benefit significantly from enhanced patient engagement and compliance, ultimately leading to higher-quality data and study success.
Implementing eCOA in clinical trials can streamline data collection and enhance the overall efficiency of your research. Here are seven key considerations for successfully implementing eCOA in your clinical trials:
Regulatory Compliance
Ensure that your eCOA system complies with the regulatory requirements of the regions where your clinical trials are conducted. Familiarize yourself with guidelines such as 21 CFR Part 11 in the United States and similar regulations in other countries.
Device Compatibility
Consider the various devices that participants may use to enter data, such as smartphones, tablets, or web browsers. Ensure that your eCOA system is compatible with these devices, taking into account factors like screen size and operating systems.
Patient Engagement
Engage with patients early in the trial planning process to ensure their comfort and willingness to use the eCOA system. Provide training and support to help participants become familiar with the technology.
Data Security
Prioritize the security of patient data. Implement robust data encryption, user authentication, and access controls to safeguard sensitive information. Regularly update security protocols to stay ahead of emerging threats.
Data Validation and Quality Assurance
Establish rigorous data validation and quality assurance processes. These should include edit checks, audit trails, and monitoring for missing or inconsistent data. Ensuring data integrity is crucial for the credibility of your trial results.
Integration with Other Systems
Consider how the eCOA system will integrate with other trial-related software, such as electronic data capture (EDC) systems and ePRO (electronic patient-reported outcomes) databases. Seamless integration can reduce data transfer errors and streamline trial management.
By addressing these considerations, you can maximize the benefits of eCOA in your clinical trials, such as improved data accuracy, increased patient engagement, and enhanced trial efficiency. However, it’s essential to adapt your approach to the specific needs and circumstances of your trial and continuously monitor and refine your eCOA implementation throughout the study.
eCOA Clinical Trials Future Trends and Innovations
eCOA (Electronic Clinical Outcome Assessment) is rapidly shaping the future of clinical trials. This innovative technology is revolutionizing data collection by streamlining the process, enhancing patient engagement, and improving data quality and integrity. With the increased adoption of eCOA in clinical trials, we can expect a shift towards more patient-centric approaches, real-time data monitoring, and a reduction in human errors. These advancements hold the potential to significantly enhance the overall success and efficiency of clinical trials, ultimately benefiting both patients and researchers.
Future trends and innovations in the field of eCOA in clinical trials:

Figure: eCOA Clinical Trials Future Trends and Innovations
Digital Biomarkers Integration
The integration of digital biomarkers in eCOA (Electronic Clinical Outcome Assessments) is expected to become a significant trend. These biomarkers, collected from wearable devices and mobile apps, can provide real-time, objective data on patients’ health and well-being, enhancing the quality and accuracy of clinical trial assessments.
Patient-Centric eCOA Design
Future eCOA solutions will prioritize patient-centric design, making it easier for patients to participate in clinical trials. This includes user-friendly interfaces, multilingual options, and customizable assessments to ensure patients’ diverse needs are met.
Real-World Evidence (RWE) Integration
To streamline drug development, eCOA systems will increasingly incorporate real-world evidence from electronic health records and other sources. This data can provide a more comprehensive view of a drug’s effectiveness and safety profile in real-world settings.
AI and Machine Learning for Data Analysis
Artificial intelligence and machine learning algorithms will play a vital role in eCOA data analysis. These technologies can identify subtle trends, predict patient outcomes, and enhance the efficiency of clinical trials by automating data quality control and anomaly detection.
Remote Monitoring and Telehealth
The COVID-19 pandemic accelerated the adoption of remote monitoring and telehealth solutions in clinical trials. eCOA systems will continue to support virtual patient visits, enabling real-time data collection, reducing the need for in-person visits, and increasing patient retention rates.
Blockchain for Data Security
Given the sensitivity of clinical trial data, blockchain technology is expected to be increasingly employed to ensure the security, integrity, and traceability of eCOA data. This will enhance data privacy, reduce fraud, and improve transparency in clinical trial operations.
Regulatory Advances
As eCOA systems become more sophisticated and widely adopted, regulatory bodies like the FDA will likely provide clearer guidance and regulations to ensure data quality, patient privacy, and the validity of eCOA in clinical trials. Staying informed and compliant with these evolving regulations will be crucial for sponsors and Clinical Research Organizations.
These trends and innovations in the field of eCOA for clinical trials are expected to reshape the way trials are conducted, making them more patient-centric, data-driven, and efficient. It’s crucial for stakeholders in the pharmaceutical and clinical research industries to stay updated with these developments to remain competitive and ensure the successful execution of clinical trials.
Embrace the Power of eCOA to Transform Your Clinical Trial Data Collection Process
Embrace the Power of eCOA to Transform Your Clinical Trial Data Collection Process. In today’s rapidly evolving landscape, hybrid clinical trials have become a pivotal component in drug development. Leveraging Electronic Clinical Outcome Assessments (eCOA) can significantly enhance the efficiency and accuracy of data collection in these trials. By incorporating eCOA solutions, you can seamlessly capture patient-reported outcomes and clinical data, ensuring a streamlined and patient-centric approach that optimizes the success of your hybrid clinical trials. The impact of eCOA on data quality and integrity is profound, offering real-time, accurate, and patient-centric data collection that enhances the reliability of clinical trial results. By replacing traditional paper-based methods with digital, patient-friendly platforms, eCOA streamlines data collection, reduces errors, and ultimately contributes to more efficient and successful clinical trials.
Conclusion
eCOA (electronic Clinical Outcome Assessment) has undeniably emerged as a game-changer in the realm of clinical trials, revolutionizing the way data is collected and transforming the entire research landscape. With its numerous advantages, including increased data accuracy, patient engagement, and cost-efficiency, eCOA has proven to be an indispensable tool for researchers and pharmaceutical companies alike. As the clinical trials industry continues to evolve, embracing eCOA is not just a choice but a necessity to enhance the quality and efficiency of data collection, ultimately expediting the development of innovative therapies and improving patient outcomes. In a world driven by technology and innovation, eCOA in clinical trials has unquestionably earned its place as a pivotal keyword in the pursuit of cutting-edge research and healthcare advancements.