Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem. The European Medicines Agency (EMA) coordinates the European Union (EU) pharmacovigilance system and sets rules and guidelines to ensure the safety of medicines marketed in the EU. As per EU law, each marketing authorization holder (MAH), national competent authority, and EMA is required to have a pharmacovigilance system in place such that the use of medicines post their approval is monitored in healthcare practice and adverse effects and safety risks are caught as soon as they occur thereby minimizing the number of exposed patients.
The EMA requires the operation of pharmacovigilance practices throughout the product lifecycle. The EudraVigilance system which is a central electronic database of adverse events is in operation throughout the product lifecycle including research and development, marketing authorization and post-authorization phases and includes activities such as safety monitoring, signal detection, risk assessment, and regulatory decision-making. Risk management plans that outline the medicine’s safety profile and assessment of the risk-benefit ratio are necessary during the marketing authorization and post-authorization phases and are mandatory to be submitted. The post-authorization phase includes several pharmacovigilance activities such European Risk Management Strategy (ERMS), adherence to Good pharmacovigilance practices (GVP), incident management plans, medical literature monitoring, medication errors, periodic safety update reports (PSURs), pharmacovigilance systems, post-authorization safety studies, and signal management.
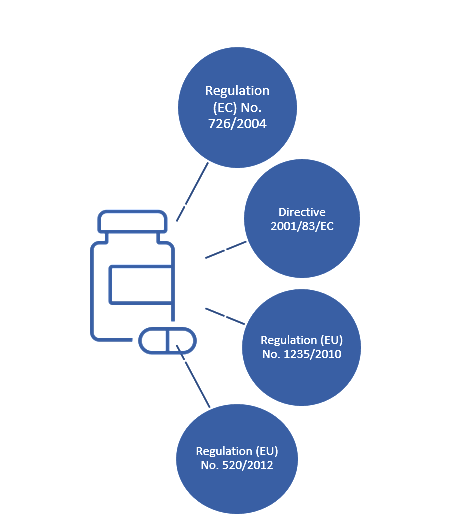
Figure 1 shows the key regulations applicable to pharmacovigilance in for medicines marketed in the EU:
Regulation (EC) No. 726/2004
This involves union procedures for the authorization, supervision, and pharmacovigilance of medicinal products for human and veterinary use. It highlights the role of the Pharmacovigilance Risk Assessment Committee (PRAC) for conduct of pharmacovigilance tasks and approval and monitoring of risk management systems. In the context of pharmacovigilance activities, it states mandatory requirements by marketing authorization holders on the continuous monitoring of medicines and post approval safety studies.
Directive 2001/83/EC
This covers the legal framework for authorization, supervision, and pharmacovigilance of medicinal products for human use in the EU. It emphasizes the importance of continuous adaption of pharmacovigilance systems, accordance with international guidelines, and on-going pharmacovigilance activities. Various pharmacovigilance activities such as adverse event reporting, risk management plans (RMPs), periodic safety update reports (PSURs), pharmacovigilance system master file (PSMF), and signal detection and management are described and should be available.
Regulation (EU) No. 1235/2010
This is related to pharmacovigilance activities for the supervision of medicinal products including RMPs, PSURs, inspections, signal detection and evaluation, ICSRs and electronic reporting, and PSMF.
Regulation (EU) No. 520/2012
This is related to the performance of pharmacovigilance activities and includes information on the content of PSMF and quality systems, information for monitoring of data in the Eudravigilance database, the signal management process, and details on the content of ICSRs and PSURs.
In November 2023, the EMA published an updated guidance on post-authorisation procedural advice for users of the centralised process which covered topics such as post-authorisation procedural advice, guidance documents and templates, pharmacovigilance activities, product information updates and timelines for submission of various activities. With respect to pharmacovigilance activities, information on changes to quality systems and PSMFs and intimation of authorities of these changes, details on pharmacovigilance system inspections, scientific advice and proactive pharmacovigilance planning procedures, submission of pharmacovigilance reports and interim reports, and additional pharmacovigilance activities are described. Guidance on post-authorization safety studies (PASS) and post-authorization efficacy studies (PAES) are also available in this guidance.
Other recent updates to pharmacovigilance activities in the EMA include
- Inclusion of real-world evidence (RWE) is emerging for the assessment of the safety of medicines in real-world settings through examination of registries and electronic health records (EHRs) and is being integrated into pharmacovigilance practices.
- Good pharmacovigilance practices (GVP) are a set of measures drawn up to facilitate the performance of safety monitoring of medicines in the EU. GVP guidelines are continuously updated to reflect changes in regulatory guidelines and advancements in safety management techniques. In 2020, GVP were modified to include requirements for submitting information on non-interventional post-authorization safety studies and inclusion of methodologies, criteria, and approaches for assessing the effectiveness of risk minimization methods.
- Electronic reporting of ICSRs and adverse effects is encouraged as it allows for submission in a standardized format facilitating information sharing, regulatory activities., and timely decision-making. Submission processes, controlled terminology, and integration with data sources are continuously changed and updated to comply with Eudravigilance databases. Latest updates to Eudravigilance system in 2022 include alignment with Substance, Product, Organization, and Referential (SPOR) data services to standardize terminologies and identifiers in data entry thereby streamlining data exchange across different stakeholders and formats.
Therefore, although the EMA has a robust system in place for pharmacovigilance activities, continuous changes and updates are inevitable and should be considered for realizing the full benefits of pharmacovigilance systems. It is important to think of pharmacovigilance as a work in progress as new data entry systems, data sources, and formats are dynamic.
Read More: Digital Health Technologies in Pharmacovigilance
References
1) EudraVigilance: electronic reporting | European Medicines Agency (europa.eu)
2) Archive of development of good pharmacovigilance practices | European Medicines Agency (europa.eu)
3) Pharmacovigilance: Overview | European Medicines Agency (europa.eu)
4) EUR-Lex – 32004R0726 – EN – EUR-Lex (europa.eu)
5) Microsoft Word – Human Code.doc (europa.eu)