Explore the dynamic synergy between industry and academia in groundbreaking medical research through Investigator Initiated Trials (IITs) and Proof of Concept Studies. Uncover the collaborative model shaping innovative partnerships, driving advancements at the intersection of industry and academia for transformative healthcare solutions.
Clinical trials remain at the centre stage of all regulatory, healthcare decisions and practice guidelines and are usually initiated and conducted by pharmaceutical companies or industry sponsors that develop the drug. Industry-sponsored trials constitute most of all trials conducted globally wherein the entire responsibility of the trial, data publication, and funding lies with the sponsors and the daily operations are carried out by investigators. Additionally sponsor-investigators play the dual role of initiating and conducting the trial. It is apparent that the sponsors’ decisions and objectives in addition to being healthcare driven are largely financially and business-purpose driven. These motives can raise issues of ethics and ultimately cast a doubt on the actual safety of the drug despite strict regulatory requirements and reporting guidelines.
In contrast to industry-sponsored trials, investigator initiated trials (IITs) are those in which the investigator who may be part of a hospital or academic institution is responsible for initiating and managing the trial. The design of the protocol, conduct of the study, and reporting of the results is done by investigators and differs from industry-sponsored studies wherein the investigator is only the executor of the study. Allowing the investigator to perform these multiple roles increases freedom in term of study design and objectives and allows for advancements in medical knowledge which may not have immediate financial benefits.
The reason that investigators have for conducting research can come from real-world experiences or observations and insights in clinic settings of certain treatments or drugs that would be generally missed if the trial was conducted for only one purpose or disease. Other reasons that IITs can help advance medical knowledge is flexibility and adaptability as the trial progresses compared to industry-sponsored trials, greater relevance to patient care based on experiences in real-world settings, more cost effectiveness due to the ability to use existing infrastructure and equipment in teaching hospitals and institutions, and independent research.
IITs have been shown to be more adept at collecting proof-of-concept data than industry-sponsored studies allowing for decisions on their continuation or termination to be made faster without expending too many resources. Some examples of IITs that have changed practice guidelines include the Anglo-Scandinavian Outcomes Trial (ASCOT)-Lipid Lowering arm (LLA) which led to a change in guidelines related to hypertension based on the reduction in coronary events by atorvastatin relative to placebo. The pulmonary embolism prevention (PEP) trial is another example of a IIT that lead several societies to recommend aspirin prophylaxis for the prevention of venous thromboembolism in patients undergoing arthroplasty. However, like all other trials, IITs must adhere to local and global regulatory guidelines, Good Clinical Practice (GCP) standards, and safety reporting, informed consent, and patient privacy regulations. Thus, the main differences between IITs and industry-sponsored studies are the funding sources, personnel conducting the studies, data publication and rights and ownership.
Although, IITs offer several advantages and may be superior compared to industry-sponsored trials in terms of expansion of product knowledge, safety concerns, and patient outcomes, they are often limited by financial constraints. The funding from IITs comes from private or government grants which is a competitive process and is of limited capacity posing cost constraints and difficulties in continuing the trial to completion in cases of cost cuts and suspension of funding. Other challenges related to regulatory oversight and submissions, data management and archival, statistical analysis, literature searches, and adequate pharmacovigilance and training programs also plague the conduct and applicability of IITs.
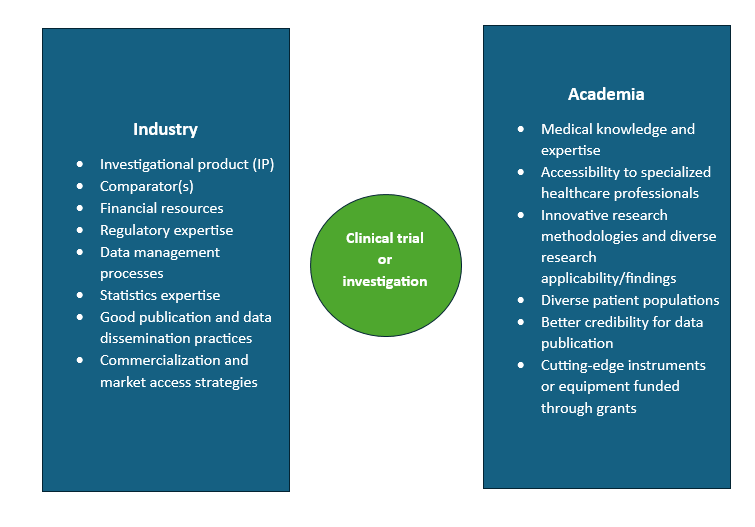
Realizing the benefits and limitations of industry-sponsored trials and IITs can help leverage the advantages of each one and develop and conduct a trial such that it is conducive to patients, is financially smooth, helps combine medical expertise from both sides, and advances medical knowledge. One of the best ways to combine sponsored trials and IITs is to form and maintain strong and mutually beneficial industry-academia partnerships. This collaboration occurs between industry-sponsors (pharmaceutical, biopharmaceutical, and medical device companies) and academia (teaching hospitals or academic institutions). The figure below denotes the different aspects to the trial brought in from industry and academia.
Thus, considering the different expertise that industry and academia bring to the table in the field of clinical research, creating and fostering these partnerships will help advance medical knowledge and improve patient outcomes. The field of clinical trials is also moving towards more observation-based or real-world approach that involves inquiring into observations made in clinical practice that can help expand patient knowledge beyond the questions that are to be answered in traditional sponsored trial settings as well as create or modify practice guidelines. IITs can achieve these objectives when the academic centers are backed and supported suitably by industry sponsors.
Read More: Clinical Trial Success: ProRelix Research’s Tips for Sponsors
References
- Investigator initiated trials (IITs) – PMC (nih.gov)
- Investigator initiated trials versus industry sponsored trials – translation of randomized controlled trials into clinical practice (IMPACT) | BMC Medical Research Methodology | Full Text (biomedcentral.com)
- Investigator-initiated studies: Challenges and solutions – PMC (nih.gov)
- Investigational New Drug Applications Prepared and Submitted by Sponsor-Investigators | FDA