Pharmacovigilance is defined by the World Health Organization (WHO) as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse events or any other drug-related problems.” Drug pharmacovigilance programs form an important part of post-marketing risk assessment and are a part of the Food and Drug Administration’s guidance on risk management activities which include the following:
- Premarketing Risk Assessment (Premarketing Guidance)
- Development and Use of Risk Minimization Action Plans (RiskMAP Guidance)
- Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment (Pharmacovigilance Guidance)
The reason why the development of appropriate and thorough pharmacovigilance plans is important is that it is impossible to capture all adverse events and safety concerns related to a drug or medical device during clinical trials that are conducted for regulatory approval. Clinical trials are carried out in patients with homogeneous characteristics under strict protocols and monitoring programs. Following regulatory approval, drugs, and medical devices are used in much larger patient populations that differ in terms of their demographic characteristics and medical conditions and which can result in safety concerns that usually do not arise or are not captured during clinical trials. Evaluation of risk-benefit information that becomes available once the product is marketed is necessary to ensure its safe use and reduce risk to patients by allowing feedback to reach users in a timely manner. Therefore, the industry and regulatory authorities are heavily focused on developing pharmacovigilance plans and safety specifications even before the drug or medical device is approved or licensed for use.
Pharmacovigilance programs involve the identification and evaluation of safety signals which refer to a concern about an excess of adverse events compared to what would be expected to be associated with a product’s use. Case reports, registries, observational studies, preclinical data, and surveys serve as potential sources of safety signals. A very important aspect of a good pharmacovigilance program is good reporting practice to ensure that safety concerns are reported and addressed appropriately. Timely reporting and communication, complete and targeted questionnaires or computer-aided interview technologies, accuracy, follow-up in relation to the seriousness of the adverse event, and confidentiality are important elements of good reporting practices and should be rigorously followed.
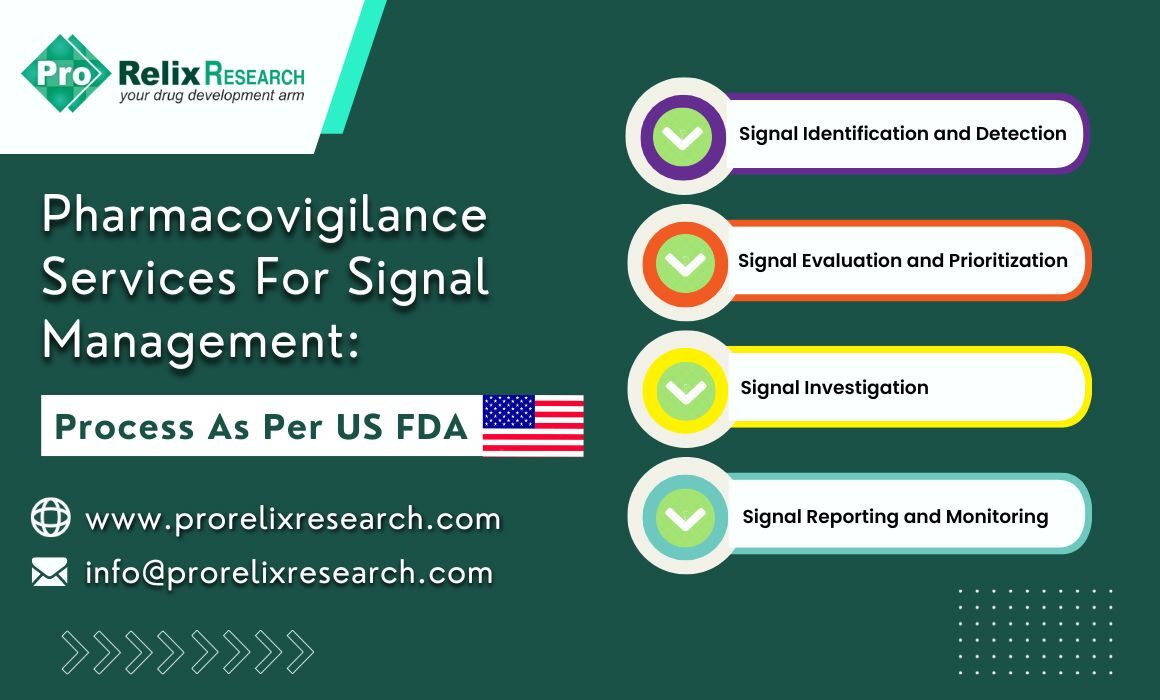
The signal management process is a set of steps that are performed to determine if there are any new risks associated with a drug or if there have been any changes to the safety profile of drugs. The figure below represents the stages for an appropriate signal management process as per US FDA guidelines:

Figure. Signal management process in Pharmacovigilance programs
Signal identification and detection
This step involves proactive searches of safety databases using data mining methods to identify any safety concerns or adverse events associated with a drug. The FDA has the Adverse Event Reporting Systems (FAERS) which includes spontaneous reports and serves as an important resource for individual case reports that are entered in by healthcare professionals, consumers, and pharmaceutical companies. The FDA has also launched the Sentinel system for active reporting of adverse events using data from electronic health records (EHRs), insurance and billing claims, and registries and provides data in real-time. Unlike the FAERS system that relies on voluntary reporting pf adverse events which may not be accurate and in some cases incomplete and biased, the Sentinel system is comprehensive. Both the FAERS and Sentinel databases are widely used in signal detection in pharmacovigilance practices. Other sources of information include observational studies, epidemiological studies, spontaneous reports, and literature searches.
Signal evaluation and prioritization
This steps focuses on understanding the reliability of the signal and determining any causal relationship between the drug and adverse event at the time of occurrence of the adverse event, presence or absence of symptoms without drug exposure, comparison of toxicity with other drugs in the same pharmacological class, and evidence of toxicity from preclinical and observational studies along with alternative reasons for the event. Although there is no definitive classification of causality, the FDA recommends categorization of causality into terms such as probable, possible, or unlikely along with a detailed justification of the classification. The FDA recommends a comprehensive account of events leading to safety concerns associated with medication errors. Data mining methods are applied to characterize and collate data. The safety concerns in case of suspected safety risks should be synthesized from various sources and should include background rate for an event, safety findings from controlled trials, measures of association such as odds ratio from pharmacoepidemiologic studies, and general marketing experience with similar products of the class. The FDA assesses potential safety risk of the signal considering factors such as strength of association between the adverse event and product, consistency of findings across available data sources, biologic plausibility, seriousness of the event, and evidence of dose-response for the effect among others.
Signal investigation and analysis
This step involves a thorough evaluation of the identified safety signal and determination of a causal relationship between the drug and adverse event. Data mining methods, and analytical methods such as odds ratio and Bayesian statistics are used to determine the relationship in the presence of confounding factors.
Signal reporting
Once the FDA has deemed a particular safety signal to pose a risk it is essential for the Agency to take suitable regulatory action which can include a change in labeling, restrictions in its use, or in extreme cases withdrawal of the drug from the market. Reporting to regulatory authorities can also be done directly by patients, healthcare professionals, and consumers through the FAERS database to help protect the well-being of patients and develop risk mitigation strategies.
The FDA has a robust system and process for identification and reporting of safety signals that is vital to protect patient welfare in the post-marketing stage. It is important for all stakeholders such as patient, physicians, regulators, and care takers to play their role and contribute to the safety of participants through a timely and accurate reporting of signals.
Read More :
Pharmacovigilance Services in cdsco
References
1) Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment | FDA
2) Guidance for Industry E2E Pharmacovigilance Planning (fda.gov)
3) FDA Adverse Event Reporting System (FAERS) Public Dashboard | FDA