Clinical trials play a pivotal role in the approval of new drugs as they most accurately reflect the effect of the drug in the human body. Since drug development is a costly and laborious process with the clinical trial phase being the lengthiest step as well as the one with the highest attrition rate, it is important to partner with experts for the conduct of clinical trials to maximize chances of success.
Clinical trials are divided into the following phases:
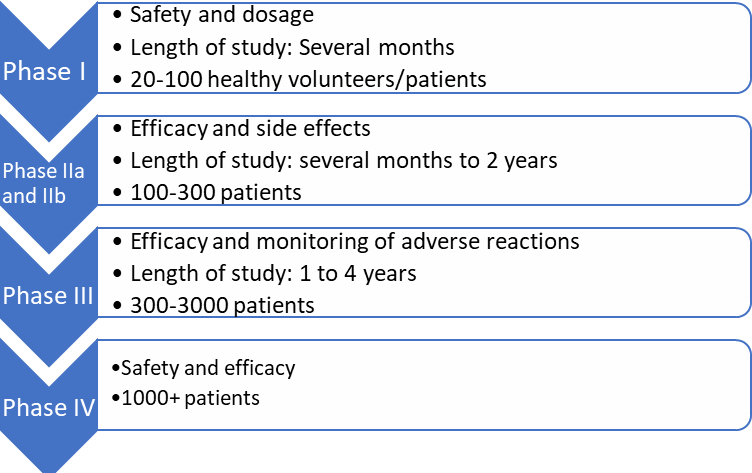
Our focus at ProRelix Research is on the science, ethics, and regulatory perspective of conducting a clinical trial:
Scientific expertize: We take the effort to understand your needs as per the drug molecule and phase of the trial and provide expert advice on trial design and statistical analysis to obtain reliable and high-quality results across a broad range of therapeutic areas.
Regulatory expertize: Our team has a strong understanding of the requirements of regulatory bodies such as US FDA, EMA, CDSCO, TGA, etc. ensuring seamless and timely regulatory submissions. We liaison with regulatory authorities and facilitate meetings to avoid roadblocks in the progress of trials.
Ethical principles: Keeping in mind participant well-being and safety as the highest priority, we adhere to our internal Standard Operating Procedures (SOPs), Good Clinical Practice (GCP) guidelines, and local guidelines to protect participant health.


We provide the following services and partner with you at every step of your trial from set-up to completion
Startup services
We help you get clinical trial ready by ensuring that everything you need to initiate a trial is available
- Document preparation: Investigator’s brochure, clinical study protocol, subject information and informed consent form, clinical study reports, case report forms (CRFs), Trial Master File (TMF)
- Site feasibility and site qualification
- Understanding the sponsor’s needs such as geographical requirements, key opinion leaders (KOLs), preferences for certain sites
- Assessment of site feasibility through questionnaires to determine suitability in terms of experience, patient recruitment, ethics, site staff, etc.
- Site qualification visits to ensure availability of equipment and infrastructure, staff, and recruitment strategies for participants
- Local regulatory approvals
- Ethics committee and Institutional Review Board (IRB)/Independent Ethics Committee (IEC) submissions
- Preparation of submission packages
- Scheduling regulatory meetings and communications with regulatory bodies
- Notification of amendments to regulatory bodies
- Negotiating site contracts
- Patient recruitment and retention strategies
- Identification of enrollment criteria and target patient populations
- Development of study awareness programs such as patient advocacy groups, community training sessions, advertisements, referral programs
- Provision of travel and caregiver support
Clinical Trial Management and Study Monitoring
We have a clinical team with expertize in project management to enable smooth functioning after trial set up. Our team of experts and Clinical Research Associates (CRA) can troubleshoot, negotiate, and make decisions during trial conduct keeping patient safety, ethical principles, and good clinical practices foremost. We also ensure compliance with global and local regulations and our team is well-versed with any changes in regulatory guidelines. Our services include:
- Establishment of quality guidelines and preparation of SOPs at the site
- Compliance with quality systems and preparation of sites for audits by conducting mock audits
- Creation of training programs such as GCP training and protocol training for site personnel
- Interim monitoring and site close-out activities
- Ensuring good documentation practices and accountability of all study materials and documents such as CRFs
- Timely communications with sponsor to provide updates on protocol deviations, recruitments, and other issues related to the trial
- Review of any deviations and safety monitoring of participants
- Accountability of investigational product (IP) such as delivery, handling, and adequate storage to ensure availability at all sites
Clinical Data Management and Statistical Analysis
Data security, accuracy, and availability are the prime focus of our data management team that is involved in ensuring efficient collection and management of data.
- Set up and validation of data collection tools or clinical databases to capture data such as electronic data capture (EDC) technologies
- Identification of key end points for data collection in protocol development
- Calculation of adequate sample size depending upon trial design to ensure statistical power
- Patient safety tracking and pharmacovigilance
- Regulatory submissions as per Clinical Data Interchange Standards Consortium (CDISC) guidelines to US FDA and EMA
- Creation and maintenance of electronic patient reported outcomes (ePROs)
- Data queries and resolution
- Database lock services and database maintenance and archival
- Statistical analysis of data and preparation of statistical analysis reports (SARs) using various statistical software programs
Need Support to Conduct your Phase 1 to 4 Clinical Trials?
We’d be delighted to assist you in conducting your Phase 1 to 4 Clinical Trials
Connect with our experienced staff today!!
Provide details of your requirements by clicking on the given link below and our professional team of clinical research experts will get in touch with you right away.




