Although time, speed, and operational constraints have long plagued the progress of clinical trials, another factor that remains important and has been gaining prime importance as the centre of clinical trials is the patient. It has been shown that patient recruitment and retention is one of the main causes for the failure of trials due to the high attrition rate of patients. Lack of communication with patients and patient awareness, poor adherence to medications due to difficultly in administration and instructions, and lack of support from clinicians and health care professionals (HCPs) are some of the reasons for high drop-out rates. In comparison to traditional site-centric trials that come with problems such as high costs of site maintenance and staff costs, patient convenience is also negatively affected. Transport costs and convenience to central sites, ability to recruit only a small subset of patients due to geographical and logistical constraints, and patient assessments and study visits at predetermined time intervals which can result in the inability to fully capture adverse events and patient concerns can hamper the success of trials. The rising accessibility and use of mobile technology, artificial intelligence (AI), and patient awareness has made patient-centric trials not only implementable but also successful in today’s world.
As patient-centric trials are focused on improving patient engagement, retention, involvement, and convenience its is imperative to involve patients in all aspects of the trial process. Clear and transparent communication with patients, data sharing, feedback forms, patient-reported outcome instruments (PRO), and patient education and awareness are the key factors that need to be focused on for the successful implementation of patient-centric trials.
One of the most common and popular trail designs that has been shaped by digital technology and remote sensors as well as the COVID-19 pandemic that caused impediments in trial progress and patient recruitment is the ‘decentralized’ approach to trials. Decentralization not only solves the problem of cost-savings to sponsors and ability to recruit and retain patients, but the model is also heavily targeted towards patient comfort and convenience. By using telemedicine and remote monitoring strategies, saving on travel times, and improving patient accessibility, trials progress smoothly and are more likely to meet their end points in addition to allowing for more robust safety monitoring and patient safety and well-being. Therefore, all these reasons point to the fact that patient-centric trials are the future of clinical trials and should be seriously considered by sponsors during the trial design and conduct phases.
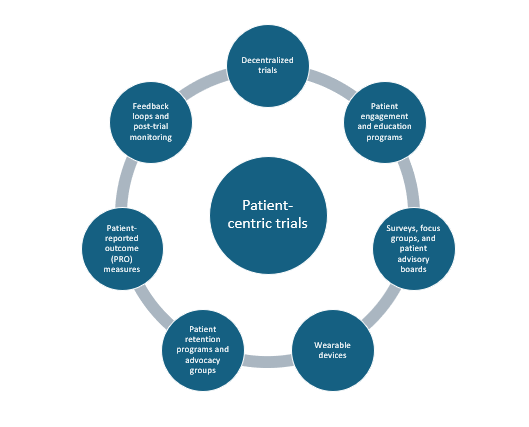
The figure below shows the various tools that sponsors/investigators can implement in the conduct of patient-centric trials.
Decentralized trials (DCTs)
As discussed, DCTs (or hybrid) form the core component of
patient-centric designs. These trials employ several tools and strategies such as remote monitoring devices, wearable sensors, and telemedicine platforms to allow for continuous and ongoing communication between patients and healthcare providers (HCPs) eliminating the need for travel to central trial sites and provide continual safety monitoring. They allow for increased patient diversity and inclusion of immobile and elderly patients in trials due to lack of geographical constraints thus allowing for more complete and reliable outcomes with broader real-world applicability. The United States Food and Drug Administration (FDA) has released a guidance document in May 2023 entitled, “Decentralized Clinical Trials for Drugs, Biological Products, and Devices: Guidance for Industry, Investigators, and Other Stakeholders” to enable sponsors/investigators to understand their perspective on DCTs and their regulatory approval standards. These efforts by regulatory agencies lend credence to the idea that DCTs are now playing a large role in clinical trial design.
Patient engagement and education programs
Putting patients foremost in trials requires that patients be involved and aware of what the trial entails, the risk-benefit profile, and be understand the informed consent process with potential safety concerns, disclaimers, and compensation information. Interactive tools such as videos and audio clippings and other digital tools in plain language without the use of scientific jargon, and proper explanations on drug administration and protocols helps improve patient retention and safety. This is an important part of patient-centric trials as it makes patients feel comfortable with the trial process.
Surveys, focus groups, and advisory boards
Considering patients and caregivers perspective is important when designing a trial. Patient advisory boards allow for patient engagement that can help shape trial design and allow for feedback from patients using PROs. Focus group discussions help to inform better clinical decision-making by HCPs and in the context of trials, group discussions can provide insights to the preferences and needs of patients. Integrating patient views facilitates patient retention in trials which is important to allow for trial completion with statistically meaningful results.
Wearable devices and other technology
Wearable sensors, watches, blood pressure monitoring devices, continuous glucose monitors, etc. help collect patient data and vital signs in real-time and transmit them to HCPs and investigators who can use this information to monitor patients’ health status and detect any untoward effects that may occur during the trial. Electronic data capture (EDC) systems also help patients report any side effects using online platforms. These devices avoid or minimize the need for patients to visit central sites and help monitor patients continually.
Patient advocacy groups
These groups help reach and inform local communities about trials occurring in their area and are useful especially for underserved communities. They help improve patient outreach, patient recruitment, and engagement and help provide real insights into treatment efficacy while always safeguarding patients’ rights.
Patient-reported outcome (PROs)
Electronic PROs are important tools to enable the collection of patients information from them directly providing a direct measure of patients’ health status including quality-of-life (QoL) measures. PROs may be
paper-based or electronic like e-diaries and apps that allow patients to fill in their information. PROs help patients to be involved in their own health and thus improve patient engagement and compliance.
Feedback loops and post-trial monitoring
These include measures that allow patients to communicate their views on the trial such as drug administration and treatment schedule, monitoring visits, and any safety issues. Feedback and post-trial monitoring systems include surveys, PROs, interviews, and focus groups. Information from these can be used to inform future trial design.
Incorporation of the above elements in clinical trial design and conduct can help shift the approach of traditional trials to a patient-centric design. The advantages of this are manifold from both the sponsors/investigators perspective to the patient. As technology advances and accessibility to online platforms and electronic systems increases it is expected that more sponsors will consider the employment of these designs and incorporate patient collaboration.
Read More: eCOA in Clinical Trials: A Game-Changer for Data Collection
References
- Decentralized Clinical Trials for Drugs, Biological Products, and Devices | FDA
- Digital Health Technologies for Remote Data Acquisition in Clinical Investigations | FDA
- Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review – PMC (nih.gov)
- Patient centric approach for clinical trials: Current trend and new opportunities – PMC (nih.gov)