Real World Evidence Studies: Introduction, Purpose, and Data Collection Strategy
The first image that comes to mind when one thinks of where safety and efficacy data for a new treatment is generated is a randomized controlled trial (RCT) at a central site. Although RCTs remain the gold standard for evidence generation of new treatments, they are limited in terms of their applicability to broader patient populations with different demographics such as age, ethnicity, and comorbidities thus limiting their generalizability. RCTs are carried out under strict conditions and dosing schedules which are often not observed in the real world, and are conducted for a limited time periods which are usually not sufficient to capture adverse events, especially in the case of chronic diseases. This has led to a shift in the thinking of sponsors, drug developers, payers, and regulators to consider the use of real-world data and real-world-evidence studies to inform decisions related to the product as well as to support reimbursement decisions.
Real-world evidence (RWE) is the clinical evidence regarding the usage and potential benefits, or risks of a medicinal product obtained from real-world data (RWD). RWD is regarded as observational data that is collected outside of a traditional RCT (1). Although the terms RWD and RWE are used interchangeably they are two distinct concepts. Not all RWD translates into RWE. RWE is obtained by detailed analyzes of data from different types of trials such as pragmatic trials, observational studies which can be prospective or retrospective, late-phase trials, or hybrid trials which are designed to collect data from patients in a real-world setting. The data that is collected can be in various forms such as electronic health records (EHRS), claims and billing data, product and disease registries, prescription data, data collected from routine hospital and physician visits, patient-reported outcomes (PROs), and mobiles and wearable devices. Recently, data from biobanks and ‘-omics’ data is becoming a valuable source of RWD. RWE studies are intended to complement data generated from RCTs by providing a detailed view of the actual use of the product and effectiveness and safety data which RCTs are unable to capture.
There has been a recent upward trend in the number of RWE trials conducted. In 2021, the Global Data Clinical Trials Database recorded 194 RWE trials and the Food and Drug Administration (FDA) published 90 examples of the use of RWE to support regulatory decisions (2). Since 2018, the FDA has released several guidances to support the use of RWD and RWE for regulatory decision-making for drugs and medical devices and the type of data to be submitted to support these applications. The two main drivers for recent interest and uptake of RWE studies are:
- Ability to gather large amounts of data using wearables, biosensors, digital platforms, and mobile devices from patients in a relatively simple and cost-effective manner that is patient-accessible and can be obtained and continuously monitored in a real-world setting.
- Improvement and sophisticated analytical capabilities allowing for the processing of large amounts of data using machine learning, artificial intelligence (AI), and data analytics.
Although the use of RWE is compelling, it is important to understand certain limitations that can compromise its use in making sound healthcare and policy decisions. Since the data that is analyzed is often reported by patients it may lack accuracy or completeness, and quality which can result in misinterpretation of data. Thus, it is necessary to design studies that adhere to certain practices related to quality that ensure reliable results. Despite this RWE studies are gaining popularity at all stages of the product lifecycle by providing data that RCTs are not able to provide.
Purpose of RWE throughout the product lifecycle:
RWE evidence studies can be used at all stages of the product lifecycle and can generate a plethora of evidence in favor of new therapies along with providing information to support clinical guidelines, regulatory decisions, and reimbursement decisions. The following figure (Figure 1) shows the various stages of the product lifecycle and how RWE studies can generate value and information across them. It is important to develop a robust and reliable data collection method and involve various business functions when developing an RWE study to maximize the potential of this study to answer several questions reflecting the real-world scenario.
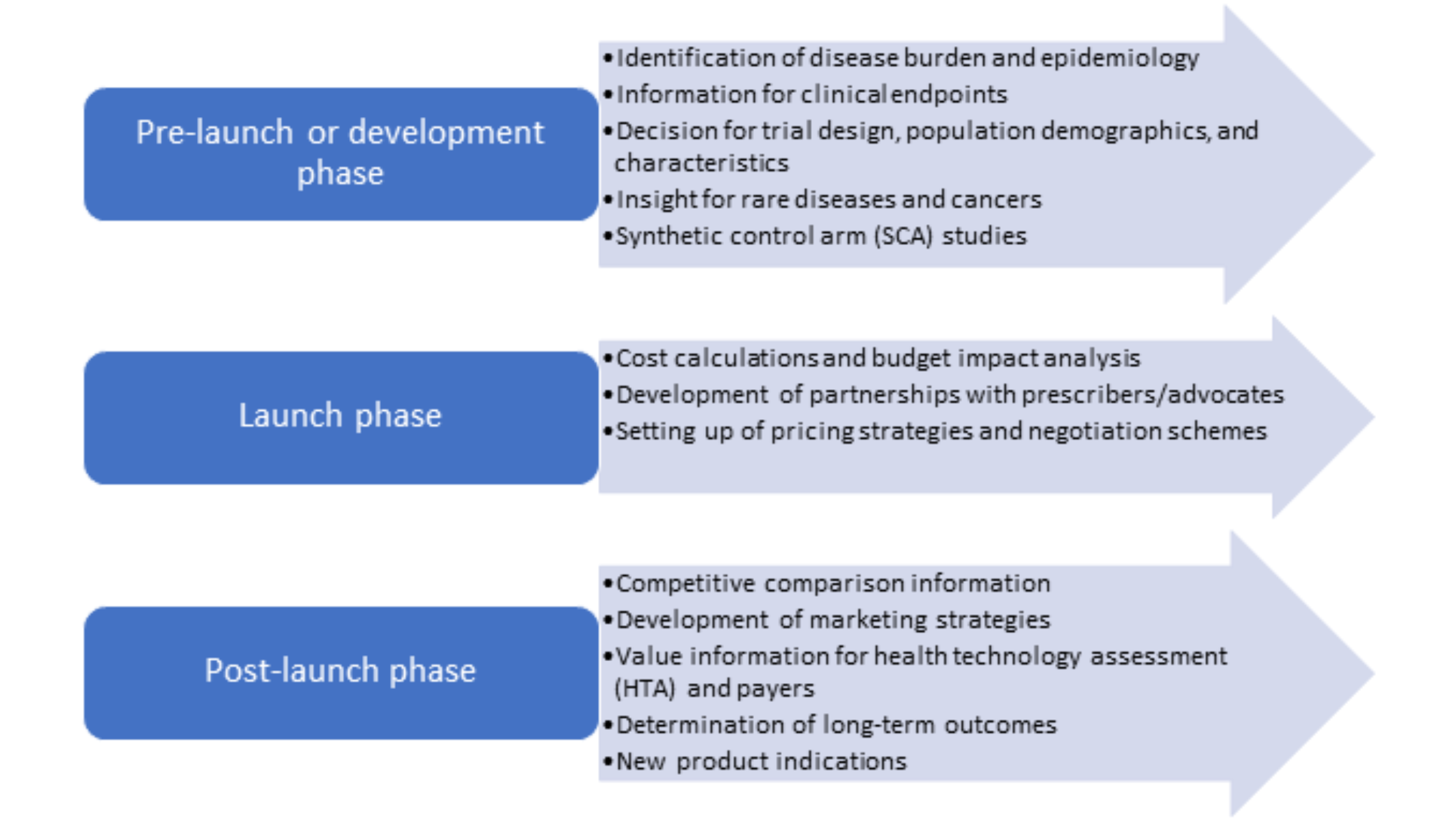 Figure 1. RWE studies throughout the product lifecycle
Figure 1. RWE studies throughout the product lifecycle
RWE can be used by all stakeholders such as researchers to provide data on long-term safety and efficacy of treatments, by regulators to assess post-marketing safety events and decide on approval, by payers to determine cost/value analysis, and by physicians to develop clinical guidelines and suitable treatment plans.
Data collection strategy for RWE studies:
There are various sources of that can be collected prospectively or retrospectively, and each serves a specific purpose to support either clinical or regulatory decisions. Some examples of RWD are:
- Healthcare databases that include electronic health records (EHR) and electronic case report forms (eCRF) which provide information on clinical outcomes and patient background and demographics. These include results of clinical and laboratory tests for patients.
- Patient registries that provide information on patient cohorts having similar characteristics such as conditions and treatments (condition-specific or product-specific registries).
- Data from patients that include social media networks and patient-reported outcome (PRO) data and patient-survey data.
- Health insurance databases that provide information on patient billing, prescribing patterns, and claims.
- Data from biobanks and ‘-omics’ data.
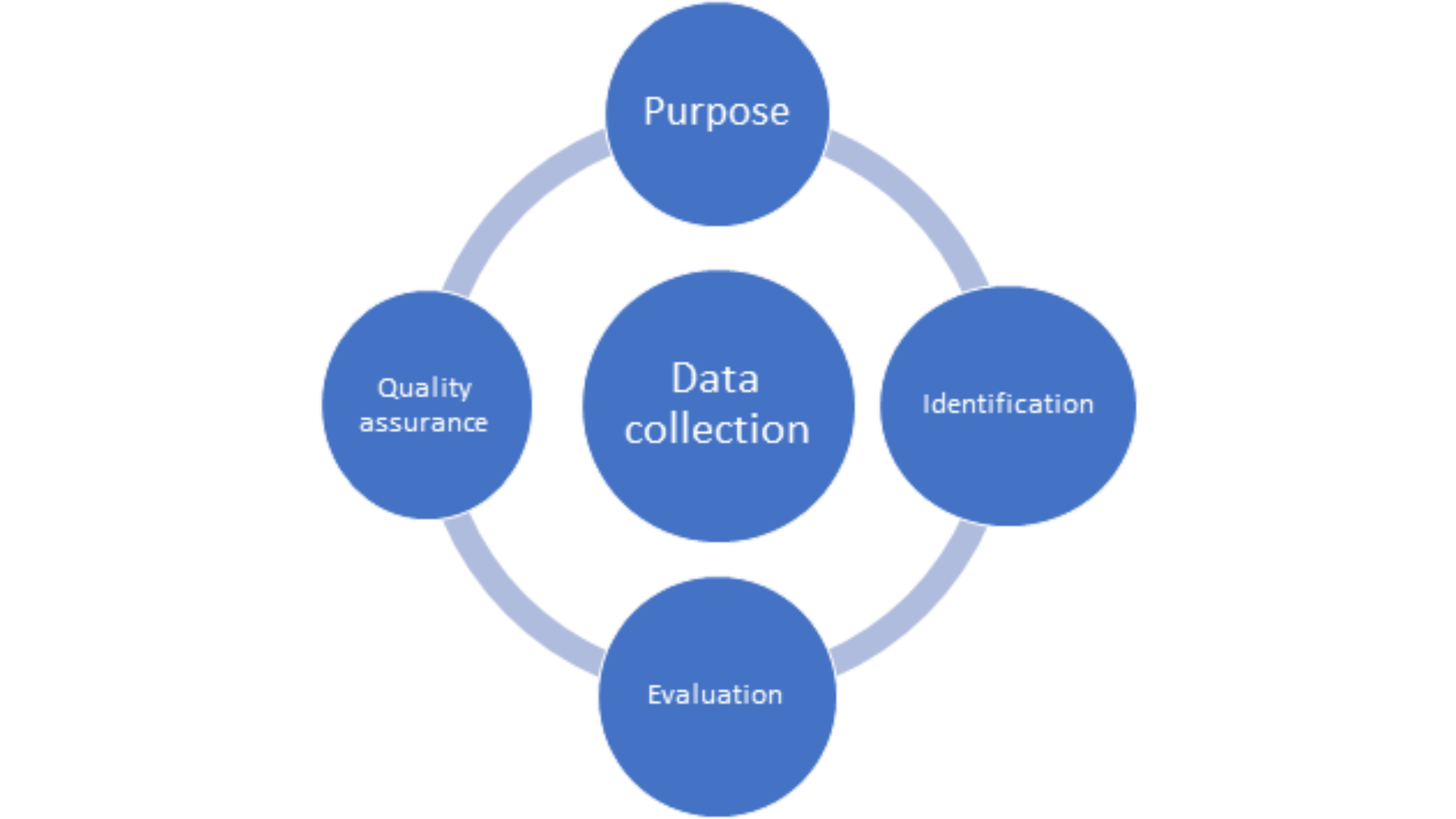
Data collection is the most critical step of any RWE study which feeds into what can be harnessed to support various decisions relating to treatment effectiveness and utilization. This is important as RWD is collected from disparate sources which are heterogeneous in terms of collection methods, language, and formats thus making it difficult to collate into a uniform format. An ideal data collection strategy for RWE studies will include the following (Figure 2):
Figure 2. Essential elements of a data collection strategy for RWE studies
- Purpose:the first step in any data collection plan involves a clear statement of the research question whether the data is to be collected prospectively or if retrospective data is to be used. It is essential that the data address the question and is fit-for-purpose. The research question must be formulated a priori before going further to collect data as each data source provides specific information. Various points such as cohort, intervention(s), outcome(s), period of data collection, and covariates should be included as part of the research question.
- Identification of data source: finding a suitable data source is the heart of the data collection process. Different data sources are used for different purposes, for example, PRO data or that from clinicians can be used to inform long-term safety or effectiveness outcomes whereas claims data can be used for HTA and economic assessments. Certain patient-specific or disease-specific registries are applicable only for certain geographical locations making it important that the data source correlates with the question that needs to be addressed.
- Data source evaluation: a systematic assessment of the data source(s) chosen involves consideration of the following factors: access and patient representation (country/region, exclusion criteria), content (patient characteristics, co-morbidities, diagnostic and prescription information, quality of life, hospital information), and costs and timelines (3). A holistic evaluation of all these factors translates into a robust data strategy.
- Quality assurance: the suitability and appropriateness for using RWD to generate evidence to support a particular purpose relies not only upon the type of data but also, it’s quality. Parameters such as reliability, transparency, accuracy, timeliness, completeness, and consistency of data must meet certain standards to assure credibility of RWE. The FDA has released guidances on checklists for assessing RWD relating to EHRs and registries (4, 5).
RWE studies are extremely valuable to pharmaceutical companies for demonstrating product value and signaling any safety concerns. However, it is necessary that RWD be collected and used prudently to ensure the reliability of RWE studies. Understanding the drawbacks of RWE in comparison to RCTs such as low internal validity and non-uniformity in data collection is necessary to appreciate the importance of both these studies in drug development. Ongoing advances in AI and data analytics, and generation and accessibility to genetic biobanks are expected to further increase the usefulness of RWE studies in all phases of the product lifecycle.
References
- Real-World Evidence | FDA
- Trends in Real-world Evidence Using Real-world Data | SDLC Partners
- Real-World Data Strategy as a Roadmap for Success | Evidera
- Real-World Data: Assessing Registries to Support Regulatory Decision-Making for Drug and Biological Products Guidance for Industry | FDA
- Real-World Data: Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making for Drug and Biological Products | FDA