Software as a Medical Device (SaMD) refers to a class of medical software intended to be used for medical purposes or that is designed to carry out medical functions without the need for actual hardware. This can comprise of software or applications intended to diagnose, cure, mitigate, or prevent disease. SaMD is a medical device or a standalone software application that is designed to complete a medical task. Examples of SaMDs are many and include:
- Mobile health apps that help patients manage their medication schedules and track vital signs (heart rate, blood pressure)
- Smart phone accelerometers to monitor balance alterations following a stroke
- Diagnostic software that uses algorithms to analyze MRI scans or X-rays for detection of tumors
- Wearable health devices such as smartwatches and fitness trackers to measure physical activity, sleep patterns, etc.
- Telemedicine platforms for consultations and physician visits
- Software to support clinical trials by collecting data for informed consent, case report forms, and safety and adverse event management
Thus, the features of SaMDs include standalone functionality such that operation is independent of hardware, application is for diagnosis, treatment, mitigation, or cure of a disease, is an independent software application, and should be regulated as a medical device as per regulatory authorities. Since technology is advancing rapidly, SaMDs are gaining popularity in healthcare settings. However, safety risks associated with SaMDs requires careful clinical investigations and evaluations and stringent regulatory oversight such that the International Medical Device Regulatory Forum (IMDRF) and the Food and Drug Administration (FDA) have developed guidance documents on quality management systems and clinical evaluation planning processes.
The clinical evaluation of SaMDs refers to the assessment and analysis of clinical data pertaining to a medical device to verify the clinical safety, effectiveness, and performance as intended by the manufacturer in the SaMDs definition statement. The level of clinical evaluation is commensurate with the risk category of the device wherein a more robust and extensive investigation plan is required for higher risk category devices. SaMDs are classified based upon the state of the healthcare situation or condition (critical, serious, and non-serious) and the significance of information provided by the SaMD to healthcare decision (treat or diagnose, drive clinical management, and inform clinical management).
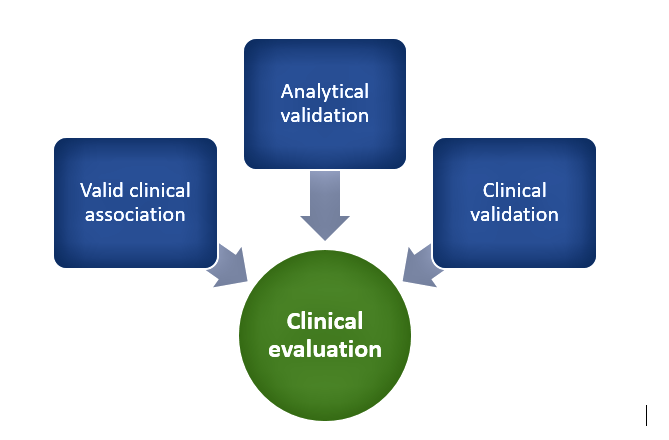
Category IV devices have a high impact and are typically used to treat or diagnose a critical condition whereas Category I devices are usually used to drive clinical management of non-serious conditions and are low impact. In addition to the degree of clinical evaluation, the need for independent review SaMDs depends upon risk posed by the device such that as per the risk-based approach, independent review of clinical evidences is most relevant for Category IV devices whereas Category I devices can suffice with self-declaration of evidence by manufacturers. In general, clinical evaluation of SaMDs includes the following as depicted in Figure 1:
Figure 1. Clinical evaluation process
Valid clinical association
This involves demonstrating that there is a valid association between SaMD output and SaMD’s targeted clinical condition through collection of evidence. This can involve existing evidence from literature searches, clinical research, and professional society guidelines or new evidence from secondary data analysis or clinical trials. It involves demonstrating that the SaMD’s output corresponds accurately in the real-world to the healthcare situation or condition defined in the SaMD statement.
Analytical validation
It is necessary to show that the SaMD correctly processes input data to generate accurate, reliable, and precise outputs and meets technical requirements. This involves demonstrating that the software meets specifications (verification) and that specifications conform to user needs and intended uses (validation). Tests and assessments are done to ensure the reliability of the results and that they fall within an acceptable range before moving onto the clinical validation phase. Quality management system activities, good software engineering practices, use of curated databases, or patient data can be used to generate evidence to support this step.
Clinical validation
This focuses on answering the question of whether the output data achieves the intended purpose in the target population in the context of clinical care and the clinical condition. Meaningful and measurable patient-reported outcomes, outcomes related to the functioning of the device, or a positive impact on health are used to determine clinically meaningful outcomes. Various statistical measures such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), number needed to treat (NNT), number needed to harm (NNH), likelihood ratios, odds ratios, and confidence intervals are used to measure clinical validity. Evidence for demonstrating clinical validity can come from that generated during quality management system development or referencing existing data from existing studies intended for similar use or extrapolation activities.
Thus, in all these steps, evidence generation lies at the heart of all clinical evaluation activities for SaMDs. The amount and depth of evidence required to support valid clinical association, analytical validation, and clinical validation activities depends upon the maturity and confidence in the evidence. Lack of evidence requires ongoing data analysis, modification of target clinical association or intended use, and changes to software to allow development of clinical associations.
In addition to the generation of evidence, a rigorous clinical evaluation process, and independent review for high-risk SaMDs, post-marketing and real-world studies form an important part of safety monitoring, on-going evidence generation and information on functionality of the device, and continuous monitoring. On-going clinical studies, direct end user feedback, and research publications allow for continuous monitoring of the device and help manufacturers modify definition statements and support intended uses.
Clinical trials for SaMDs may be required as part of the clinical evaluation process to provide evidence to support device use and conformance with regulatory requirements. In line with clinical trials for medical devices the requirements for trials for SaMDs include the following: protocol development, regulatory approval, patient recruitment, informed consent, trial execution, data collection and analysis, safety monitoring, trial completion and close-out activities, and regulatory submission of collected data. High-risk devices are subject to more extensive clinical trials than lower risk devices.
In conclusion, understanding your device and software requirements, its risk category, and a robust evidence generation plan are crucial for SaMD approval by regulatory authorities. Furthermore, commitment to well-designed post-marketing studies is necessary for understanding real-world use and if necessary to make changes to design, labeling or safety information. Continuous communication with regulatory authorities is necessary to develop appropriate strategies for demonstrating usefulness of SaMDs and necessary studies for marketing approval.
Read More: FDA Guidance on Advancement of Decentralized Clinical Trials
References
- Software as a Medical Device (SAMD): Clinical Evaluation | FDA
- Final Document: Software as a Medical Device (SaMD): Clinical Evaluation (imdrf.org)