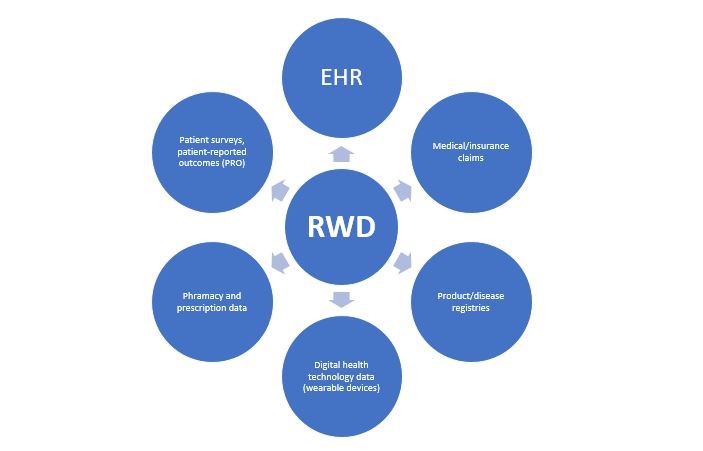
The increasing focus and acceptance of real-world data (RWD) in clinical decision making has led regulatory authorities and sponsors to understand its influence on data management that is an integral part of the clinical trial process. The United States Food and Drug Administration (FDA) defines RWD as data relating to patient health status and/or delivery of healthcare routinely collected from a variety of sources and real-world evidence (RWE) is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD. There are various sources of RWD as shown in Figure 1
Figure 1. Sources of real-world data (RWD)
The FDA has also released several guidances that describe best practices for data standards, safety and efficacy determinations, and documentation standards for use of RWD and RWE in regulatory decision-making.
Although the benefits of including RWE in supporting regulatory decisions is important as it provides information on the safety and effectiveness of a product in real-life settings and diverse patient populations, allows for collection of data for a longer follow-up time than traditional clinical trials, and provides a comprehensive view on safety risks, and labelling recommendations, it is evident that the variety of data sources would create challenges in data management. Inconsistent data formats and vocabularies, differences in terminologies, methods, and algorithms to create datasets, and data privacy issues affect the reliability and accuracy of data and need to be addressed prior to the inclusion of RWE as a part of clinical data. The FDA recommends data transformation, conversions, and mappings of data to a standardized format, discussions with FDA when planning to use RWD, and application of Clinical Data Interchange Standards Consortium (CDISC) data standards such as Study Data Tabulation Model (SDTM) to RWD sources prior to regulatory submission.
As the primary difference in data generation and collection from controlled clinical trials and real-world settings relates to diversity and inconsistencies, it is important that CDM practices account for this difference and have processes in place to deal with it. The following are the ways in which RWE impacts CDM:
Data diversity
Collection of data from various sources with inconsistent formats requires a suitable data collection strategy or tool to cover all areas of data collection whether it be from registries or wearable technology.
Data volume
Since data is collected from diverse sources and from a significantly larger number of participants than traditional trials with varying data fields, it is reasonable to assume that the volume of data collected is considerable and is known as ‘big’ data. Dealing with this tremendous amount of data requires artificial intelligence (AI) and advanced analytics methods to retain data that is essential and extract suitable relevant information from it.
Data standardization and transformations
This is necessary to ensure the data are in consistent and reliable format in accordance with CDISC or other standards. This facilitates data sharing and ensure high-quality data especially when it is from disparate sources. Data cleaning and harmonization efforts are a significant
Data privacy and security
Collection of real-world data from wearable health technology and sensors improves patient convenience and allows for real-time monitoring. However, sharing sensitive personal information on apps and other web-based devices can cause a breach of privacy. Thus, it is critical to develop suitable security protection services and privacy regulations such as Health Insurance Portability and Accountability Act (HIPAA) in place when using electronic data exchanges in clinical trials and to remove identifiable information from datasets.
Data partners
RWE studies utilize information that is reported directly from patients using questionnaires, surveys, and patient-reported outcome (PRO) instruments which tend to be subjective and ambiguous thereby creating confusion and lack of trust in the collected data. Thereby it is important that Clinical Data Management services focus on the development of clear and simple PRO forms that are easy to understand and extract information from when entered in by patients and caretakers allowing for accurate data and improved patient engagement.
Regulatory modifications
Utilization of RWE to support regulatory decisions requires that RWD to be integrated into applications and regulatory submissions in the format that is required for a particular country. Therefore, CDM professionals need to be trained to understand the requirements and ensure that the data meets the format needed.
Type of trials
Most trials use RWD along with data from traditional clinical trials for drug approval processes requiring CDM personnel to be able to handle and utilize both types of data effectively.
Therefore, although RWE brings a plethora of information from the real-world, it is necessary to employ appropriate techniques for data handling, standardization, and presentation such that the information can be used effectively to support health-care decisions. CDM professionals and staff should be able to handle diverse and high-volume data by employing analytical tools for data extraction and understanding information for regulatory applications.
Read More: Ophthalmology disorders and current developments of novel treatments under clinical trials
References
- Real-world data: a brief review of the methods, applications, challenges and opportunities | BMC Medical Research Methodology | Full Text (biomedcentral.com)
- Real-World Evidence | FDA
- download (fda.gov)
- JCI – Opportunities and challenges in using real-world data for health care