Introduction to The Role of Real-World Evidence (RWE) in Drug Development
The Food and Drug Administration (FDA) defines real-world evidence (RWE) as the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD) that includes data derived from electronic health records (EHRs), patient health surveys, medical claims data, data from product/disease registries, and data gathered from digital health technology products such as wearable sensors. Based on this definition, it is easy to see that real-world evidence (RWE) brings a different perspective to drug development compared to the traditional clinical trial paradigm. Clinical trials are conducted under regulated conditions and in relatively homogenous patient populations for a limited period, thus it is not uncommon to miss adverse events or safety issues as well as off-label uses of the product that can only be captured when the product is marketed and used in a broader patient population.
Therefore, RWE forms a critical component of the drug development process and is picking up speed largely due to internet access, availability and affordability of remote sensors, and digital technologies (DHTs) such as telemedicine platforms. There is a plethora of advantages of RWE studies such as increased generalizability, higher external validity, improved understanding of long-term implications of the treatments, reduction in drug approval costs, detailed analysis of the product/treatment in certain patient subgroups such as those with comorbidities, and accelerated research findings and knowledge. Therefore, RWE studies should ideally go hand in hand with randomized controlled trials (RCTs) to delve into and provide deeper insights into product effectiveness and safety in certain patient subgroups.
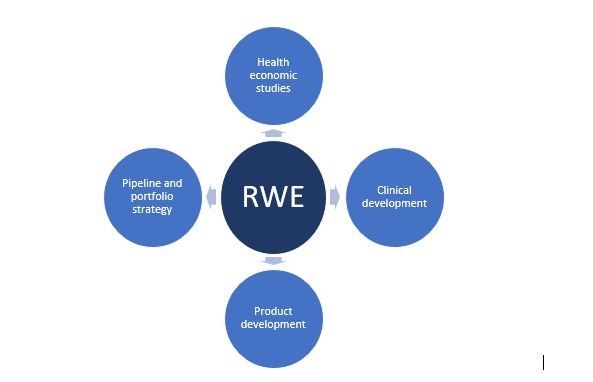
Although RWD and RWE is mainly used to guide regulatory decisions by allowing for product approval based on single-arm trials and provide post-marketing safety information and new product indications, its application is broader to several steps in the drug development program. Thus, there is a shift in the application of RWE in only the post-approval phase to include also various phases in the approval process which is evident from the publication of several guidance documents and initiatives by global regulatory authorities on the development, use, and submission of RWE for decision-making. Additionally, it was seen that RWE was used to develop protocols and safety measures such as mask wearing and use of certain medications during the recent COVID-19 pandemic highlighting its use in the approval of products for emergency use situations. The figure below shows the different aspects in the drug/product development process in which RWE can be used to guide decisions on the efficacy and safety of the product.
Figure. Application of RWD/RWE studies in Drug Development
Health Economic Studies
RWE studies assess the impact of a product on health resource utilization, cost-effectiveness, and value are often conducted post-approval. These data are extracted from registries, claims databases, and EHRs and can be used for value proposition statements and comparative effectiveness studies between proposed/novel treatments and standard of care. RWD can be used to obtain model parameters allowing for the prediction of health care costs and resources in a diverse patient population representative of real-world settings by providing data on patient compliance and adherence, long-term outcomes and survival, and direct and indirect costs associated with a treatment and disease.
Clinical Development
RWE studies can be used to leverage the development of several aspects of clinical studies such as selection of trial design, identification of diverse patient populations, trial execution, and selection of surrogates and end points. As several clinical trials fail or are unable to be completed because of these factors, RWE studies can help provide answers to key questions in the development of clinical trials thus optimizing their performance and success. RWD can be used to inform decisions on the incidence and prevalence of a disease in certain populations which can then help select target subgroups that are most likely to benefit from the treatment. These data can also help identify geographical clinical trial sites to facilitate adequate patient recruitment in areas with a high disease prevalence which can help overcome the hurdle of patient enrolment which is often a roadblock in clinical trial performance. Patient-reported outcomes (PROs) that are used in RWE studies can help in the identification of suitable clinical or surrogate endpoints for trials that have both clinically meaningful and patient-centric outcomes. RWD also plays an important role particularly in rare disease trials where sufficient data may not be available from patients under controlled trial settings to help serve as control arms. Overall, RWE can help companies/sponsors design better, pragmatic, and relevant clinical trials.
Product Development
Continuous monitoring of patients’ post-approval of treatments/products is a components of RWE studies and provides an insight into adverse event and safety issues that can be used iteratively to refine the product and improve it over time especially in certain patient subgroups. The diversity of data that is collected from PRO instruments, wearable sensors, and remote technologies that is used in RWE studies can help refine the product and proactively address problems that may exist based on its performance. Additionally, data analytical techniques can be used to extract data that can provide information on new indications of the product based on its usage in real-world settings and modify labelling indications.
Pipeline and Portfolio Strategy
RWE studies can be used in developing and refining target product profiles (TPPs) during product development to help decide upon target indications based on disease incidences and prevalence as well as potential drug interactions and safety issues that can arise in cases of renal and hepatic impairment in patients. RWD from claims data can be used to develop market access, reimbursement, and pricing strategies in certain subpopulations and can also be applied to lifecycle management strategies allowing for product differentiation, generic competition, patent protection. RWE studies can provide insight into broader indications that can support label expansions and new indications allowing for the company to increase its market potential.
As described, RWE studies have an immense impact on all states of drug development and it is important for companies to harness the potential of RWD to make better decisions from a clinical, regulatory, safety, and marketing perspective.
Read More: The Impact of Real-World Evidence on Clinical Data Management
References
2) Real-World Evidence—Current Developments and Perspectives – PMC (nih.gov)
3) Real world evidence: An Indian perspective – PMC (nih.gov)