The growing impetus for the conduct of clinical trials outside the United States for products and medical devices intended to be used in the US include multiple reasons such as increased accessibility to patient populations particularly in case of rare diseases, faster enrolments, reduced operational and clinical expenses, ethical or legal reasons, shorter trial timelines, and expertise in certain therapeutic or disease areas in other countries through the use of contract research organizations (CROs). These advantages have prompted sponsors to use clinical data from foreign sites conducted under an Investigational New Drug (IND) application and non-IND foreign sites to support clinical investigations or marketing approvals in the U.S. As per legal requirements, the Code of Federal Regulations (CFR), 21 CFR 316.106 (for drugs) and 21 CFR 814.15 (for medical devices), the following criteria must be fulfilled for foreign clinical data to be used to support marketing approvals in the U.S.:
- The foreign data are applicable to the U.S. population and U.S. medical practice;
- The studies have been performed by clinical investigators of recognized competence;
- The data may be considered valid without the need for on-site inspection by the FDA or, if the FDA considers such an inspection necessary, FDA is able to validate the data through an on-site inspection or other appropriate means.
Thus, ensuring the applicability and extrapolation of the data U.S. populations and adhering with regulatory conditions is vital when data from foreign clinical trials is used. However, challenges such as proper documentation standards and data quality, regulatory considerations, investigational product (IP) supply, safety monitoring, and personnel training are some issues that continue to plague foreign clinical trials. Of these, lack of data quality, accuracy, consistency, and completeness are the most common reasons for difficulties in using foreign data. The FDA has high standards and expectation for data that must comply with certain guidelines and regulations which may be hard to fulfil as the data collection practices and monitoring strategies maybe different in countries outside the U.S.
The FDA has released a guidance document entitled ‘FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND Frequently Asked Questions’ in March 2012. This guidance states that foreign studies conducted under an IND must comply with the requirements of the FDA for IND investigations. However, sponsors that do not choose to conduct a study under an IND must comply with the regulations under 21 CFR 312.120 which states the conditions for acceptance of foreign studies. In addition to CFR regulations, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E17 guidelines also provide information on conduct and data required from multi-regional clinical trials (MRCTs) to prevent duplication and provide bridging information to extrapolate data from one region to another taking into account ethnical and regional factors.
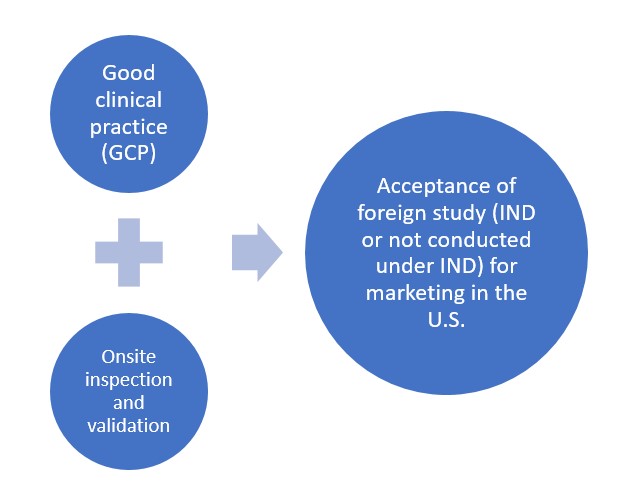
According to 21 CFR 312.120, FDA will accept support for an IND or application for marketing approval for a study not conducted under an IND if it is well-designed and conducted and if the following conditions as shown in the Figure below are met:
Figure. FDA conditions for acceptance of foreign clinical studies
Good Clinical Practice (GCP) guidelines
FDA requires that trials conducted for products intended to be used in the U.S. comply with GCP standards to ensure that the data collected are credible, accurate, and ethically procured. Compliance with GCP standards is necessary for protecting the rights and well-being of study participants by documenting informed consent that are required to be approved by independent ethics committees (IEC) that oversee that the trial is conducted under ethical considerations. Necessary data that is necessary for FDA approval is informed consent documentation, local regulatory approval, case report forms (CRF), detailed study protocols, data on safety events and adverse events reporting, statistical analysis plans and clinical study reports, quality assurance documentation such as standard operating procedures (SOPs) followed, audit and inspection reports, protocol deviations and their reasons, and documents to show Good Clinical Practice (GCP) training of personnel and documentation for compliance with GCP standards. Furthermore, supporting information should include a description of investigator’s qualifications and research facilities and chemistry, manufacturing, and controls (CMC) related to the drug substance and drug product. It is also required of the manufacturer/sponsor to delineate the location of the trials and provide data and documents on how the trials conformed with GCP regulations. FDA recommends the use of the electronic common technical document (eCTD) format with a submission letter to list out data in support of a particular clinical trial phase.
Onsite inspection and validation
Onsite inspection and audits are important when clinical trials are conducted outside the U.S. to ensure compliance with regulatory requirements and data standards that are necessary to obtain marketing approval in the U.S. Audits are done for quality assurance purposes and to protect the integrity of the participants. The probability of an audit increases when FDA has a reason to suspect fraud, mishandling of data, or violation of patients safety and welfare. It is necessary to have audit-ready documents and reports available during inspections as well as audit certificates to facilitate quick and effective results and allow the auditors to assess where the data is insufficient or unreliable. Thus, data must be at hand during inspections and maintained systematically as per U.S. data standards.
Although conducting foreign trials may be a lucrative and practical option for U.S. manufacturers/sponsors, it is necessary that the data generated is up to U.S. regulatory standards if the product is to be marketed in the U.S. Along with audits, inspections, and GCP compliance, the FDA encourages pre-submission meetings between the sponsors and regulatory authorities to allow the trials to proceed smoothly.
Read More : FDA’s Clinical Data Standard Requirements for Clinical Trials
References