The post-pandemic clinical trial landscape has paved way for the integration of technology, data analytics, and digitization to facilitate all aspects of trial management from patient identification and recruitment to data collection and statistical analysis. Decentralized, site-less, and virtual trials are becoming increasingly popular based on their several advantages such as:
- Improved patient recruitment: the ability to reach out and recruit patients that are geographically distant from the trial location thereby increasing patient diversity and number
- Increased patient retention and compliance: reduced need to travel and reminders to fill in outcomes information through SMS or e-mail reminders
- Reduces site maintenance and staff costs due to their virtual nature
- Increased data quality as information is entered in a timely manner which allows for continuous and real-time monitoring of efficacy and safety events
- Ability for seamless progress of the trial independent of global issues and travel restrictions
In addition to the shift from physical to virtual approaches to clinical trials, another aspect of trials that is evolving is ‘patient-centricity’. Patient-centric approaches involve considering both the convenience of patients such as their travel time and loss in productivity that may be incurred due to travel to trial sites as well as their feedback that can help guide trial management and safety monitoring. Perspectives from patients regarding the drug or medical device can support physician preferences and payer reimbursement issues and help regulators make more sound and robust approval decisions. Patient feedback also forms part of real-world data (RWD) and real-world evidence (RWE) through the processing of data obtained from patient registries, patient surveys, and questionnaires. Thus, the amalgamation of patient-centricity and technology has led to the use of information obtained directly from patients for making decisions on the safety and efficacy of experimental drugs and medical devices in clinical trials.
Clinical outcome assessments (COA) and electronic COA (eCOA)
The United States Food and Drug Administration (FDA) defines a clinical outcome assessment (COA) as a measure that describes or reflects how a patient feels, functions, or survives. There are various types of COAs depending upon the person reporting the patients’ symptoms, but the fundamental focus is on patient health status (Figure 1). The various types of COAs are as follows:
Patient-reported outcome (PRO) measure:
measurement based on a report comes directly from the patient/study subject about the status of the patient’s health condition without any amendment or interpretation by a clinician or anyone else.
Observer-reported outcome (ObsRO) measure:
measurement based on a report of observable signs, events, or behaviors related to a patient’s health condition by someone other than the patient or health professional such as a parent, caregiver, or someone who observes the patient in daily life.
Clinician-reported outcome (ClinRO) measure:
measurement based on a report that comes from a trained healthcare professional after observation of a patient’s health condition through interpretation of observable signs, behaviors or other manifestations related to the disease or condition.
Performance outcome (PerfO) measures: measurement based on standardized task(s) actively undertaken by a patient according to a set of instructions.
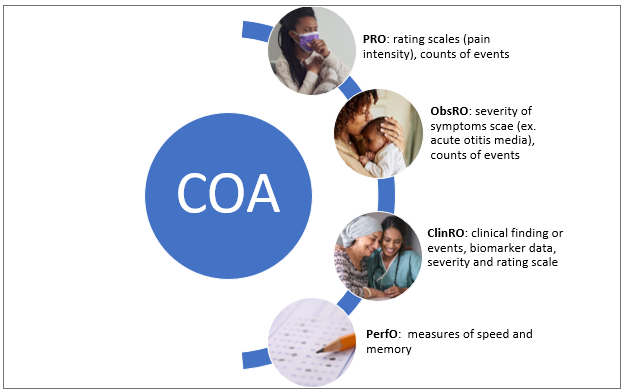 Figure 1. Types of Clinical Outcome Assessment (COA)
Figure 1. Types of Clinical Outcome Assessment (COA)
Initially, most COAs were paper-based wherein the data entered by the patient, caregiver, or clinician on paper forms were transcribed manually onto electronic databases. This caused several errors in data transfer, issues with data security, and inaccurate information as the data was not usually entered by the patients at the time of occurrence of signs/symptoms. The advent of technology, improved availability and accessibility to the internet, and increased patient comfort with the use of mobile technology have led to the uptake of electronic COAs in clinical trials. An eCOA is a digital version of a COA and is used to measure the efficacy and safety of health interventions. Various technologies such as mobile devices (smartphones, tablets), computers, interactive voice response systems (IVRS) are deployed to capture information about a patient’s health status and can be in the form of ePRO, eObsRO, eClinRO, and ePerfO. Advantages of COAs include:
- Time-savings as they eliminate the steps associated with transferring information entered on paper records to databases which are often error-prone
- Improved patient compliance through patient alerts and reminders
- Improved data quality as the data is entered by the patients in a timely manner and not just at the end of the trial or before a visit
- Data completeness through mandatory fields and validation checks that allows for more thorough and complete data capture
- Allows for real-time data capture and monitoring of adverse events
- Improved regulatory compliance
Patient-reported (PRO) measures and electronic Patient-reported Outcome (ePRO)
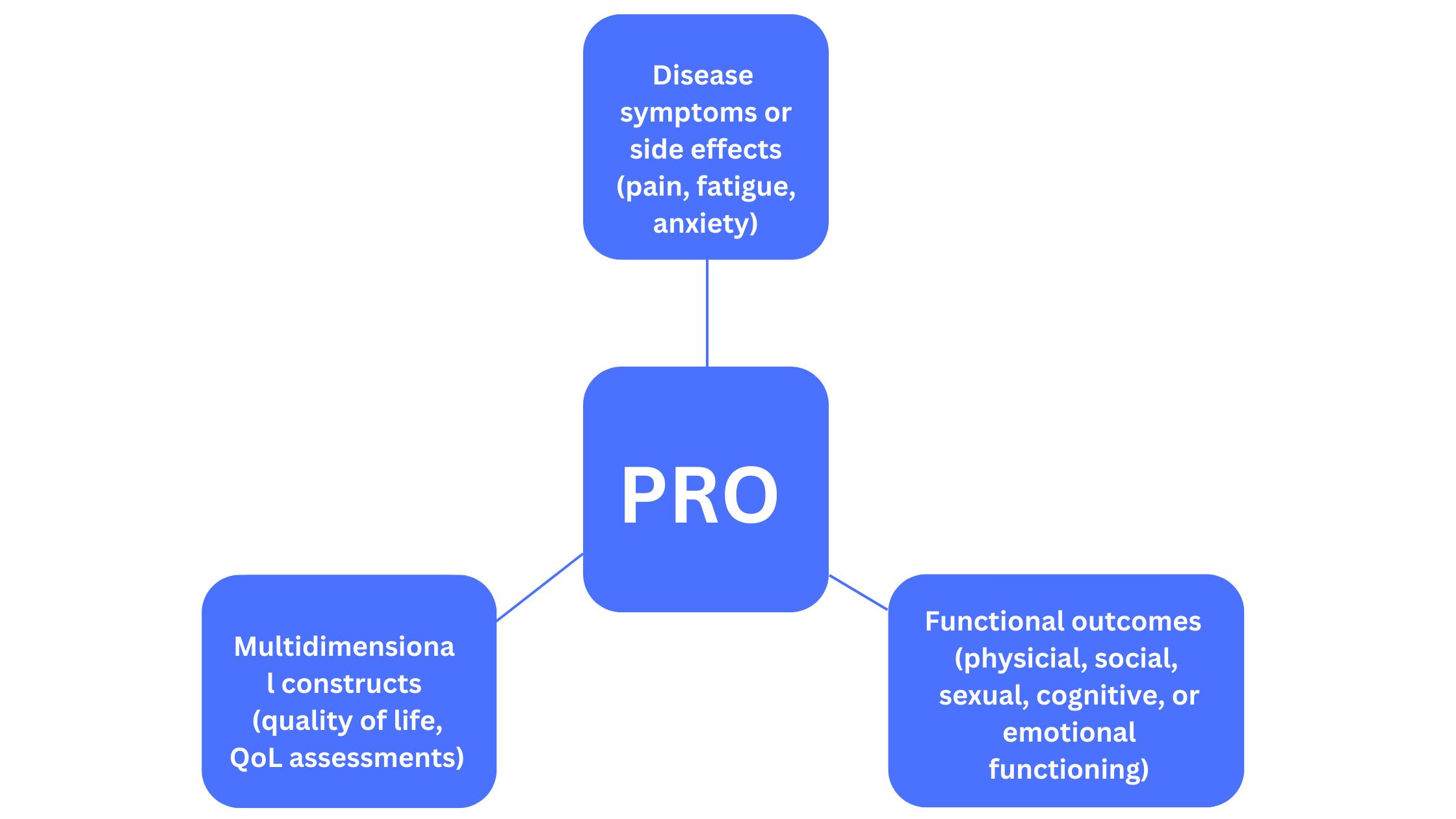
Patient feedback and experience with healthcare interventions are important as they form an integral part of RWE which is being looked upon by regulators as an essential component of data that aims to monitor the safety and efficacy of treatments. There are various types of PROs that refer to different endpoints (Figure 2).
Figure 2. Various measures by PROs
PRO instruments include patient diaries and validated questionnaires that are used to capture data directly from patients and can be in the form of ePROs. PRO measures are usually developed with input from various stakeholders such as clinicians and psychometric experts to ensure that the questions address issues in an objective manner without any bias.
Electronic patient-reported outcome (ePRO) measures allow data to be entered directly by patients in an electronic format. Like other eCOAs, ePROs also have certain distinct advantages over traditional PRO measures such as:
- Real-time data capture which is particularly important in case of pain symptoms and safety events that require immediate attention
- Better patient adherence because of easy-to-fill questionnaires that allow for data entry at the time of occurrence
- Improved patient convenience as data can be collected from technologies such as smartwatches and is transmitted directly to research teams without any change in the patient’s daily activities
There are different types of ePROs such as:
- Electronic questionnaires that periodically assess patients’ health status at predetermined times set by the clinical trial protocol on tablets, computers, and mobile devices
- Patient diaries or activity logs that involve data entry by the patient at any time convenient to them which can help detect safety issues as soon as they occur
- Fitness and health trackers that automatically record data without any intervention from the patient
- Ecological momentary assessment (EMA) that assesses a patient’s experiences, behaviors, and moods as they occur in real-time
Thus, technological advances combined with the COVID-19 pandemic have increased the uptake of eCOAs and ePROs to capture patient data that can help make better and more robust decisions about newer treatments. Despite the obvious advantages of these
patient-centric and remote assessments, it is essential for the sponsor and clinical trial team to determine the type of outcome that is necessary for a particular trial and choose a suitable measure to capture this. Furthermore, it is also extremely important to use a validated measure and ensure that the platform for data collection whether it be a smartphone, tablet, or computer can be suitably integrated with the eCOA system. It is the hope that eCOA will help reduce patient burden and improve patient experience by the elimination of tedious paper-based data collection methods.
References
- Clinical Outcome Assessment (COA): Frequently Asked Questions | FDA
- Patient-reported outcomes: A new era in clinical research – PMC (nih.gov)
- The importance of patient-reported outcomes in clinical trials and strategies for future optimization – PMC (nih.gov)
- Why Aren’t All Pro/Coa Clinical Trials Using Electronic Data Collection To Optimize Data Integrity And Patient Experience? – Value in Health (valueinhealthjournal.com)